Hint: The energy released will be equal to the change in surface energy.
Step 1: Find the change in surface area.
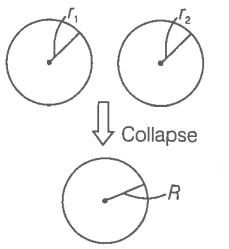
Consider the diagram.
Radii of mercury droplets,
Surface tension, (T) = 435.5 xN/m
Let the radius of the big drop formed by collapsing be R.
The volume of big drop=Volume of small droplets
or
or R =
Change in surface area,
Step 2: Find the change in surface energy.
The energy released (where T is the surface tension of mercury)
(The negative sign shows absorption)
Therefore, 3.22 J energy will be absorbed.