Define ionisation enthalpy. Discuss the factors affecting ionisation enthalpy of the elements and its trends in the periodic table.
|
Element of 2nd period |
Li |
Be |
B |
C |
N |
O |
F |
Ne |
|
Nuclear charge |
+3 |
+4 |
+5 |
+6 |
+7 |
+8 |
+9 |
+10 |
|
First ionisation enthalpy (kJ mol-1) |
520 |
899 |
801 |
7086 |
1402 |
1314 |
1681 |
2080 |
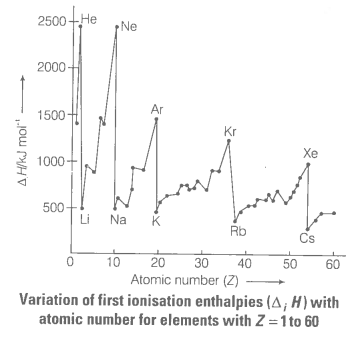
(ii) Atomic size or radius Ionisation enthalpy decreases as the atomic size increases. As the distance of the outer electrons from the nucleus increases with increase in atomic radius, the attractive force on the outer electron decreases.
As a result, outer electrons are held less firmly and hence lesser amount of energy is required to knock them out. Thus, ionisation enthalpy decreases with increase in atomic size. Ionisation enthalpy is found to decrease on moving down a group.
|
Element (alkali metals) |
Li |
Na |
K |
Rb |
Cs |
|
First ionization enthalpies (kJ mol-1) |
520 |
496 |
419 |
403 |
374 |
(iii) Penetration effect of the electrons Ionisation enthalpy increases as the penetration effect of the electrons increases. It is well known fact that in case of multielectron atoms, the electrons of the s-orbital has the maximum probablility of being found near the nucleus and this probability goes on decreasing in case of p, d and f-orbitals of the same shell.
In other words, s-electrons of any shell are more penetrating towards the nucleus than p-electrons the same shell. Thus, within the same shell, the penetration effect decreases in the order s<p<d<f
e.g., First ionisation enthalpy of aluminium is lower than that of magnesium. This is due to the fact that in case of aluminium , we have to pull out a p-electron to form Al+ ion whereas in case of magnesium we have to remove an s-electron of the same energy shell to produce Mg+ ion.
(iv) Shielding or screening effect of inner shell electrons As the shielding or the screening effect of the inner electrons increases, the ionisation enthalpy decreases. Consequently, the force of attraction by the nucleus for the violence shell electrons decreases and hence the ionisation enthalpy decreases.
(v) Effect of arrangement of electrons If an atom contains exactly half filled or completely filled orbitals then such an arrangement has extra stability. Therefore, the removal of an electron from such an atom requires more energy than expected.
e.g., Be has higher ionisation enthalpy than B and N has higher ionisation enthalpy than O . In general, as we move from left to right in a period, the ionisation enthalpy increases with increasing atomic numbers.
The ionisation enthalpies keep on decreasing regularly as we move down to a group from one element to the other.