4.12 HPO can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing HPO ? If not, give reasons for the same.
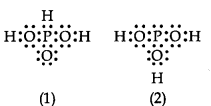

© 2026 GoodEd Technologies Pvt. Ltd.