Electronic configuration of carbon atom:
In the excited state, the orbital picture of carbon can be represented as:

Hence, carbon atom undergoes hybridization in molecule and takes a tetrahedral
shape
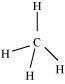
For a square planar shape, the hybridization of the central atom has to be dsp
.
However, an atom of carbon does not have d-orbitals to undergo dsp hybridization. Hence,
the structure of CH cannot be square planar.
Moreover, with a bond angle of 90° in square planar, the stability of CH will be very less
because of the repulsion existing between the bond pairs. Hence, VSEPR theory also
supports a tetrahedral structure for CH.
4.22 Explain why BeH molecule has a zero dipole moment although the Be–H bonds are polar.
Electronic configuration of carbon atom:
In the excited state, the orbital picture of carbon can be represented as:

Hence, carbon atom undergoes hybridization in molecule and takes a tetrahedral
shape

For a square planar shape, the hybridization of the central atom has to be dsp
.
However, an atom of carbon does not have d-orbitals to undergo dsp hybridization. Hence,
the structure of CH cannot be square planar.
Moreover, with a bond angle of 90° in square planar, the stability of CH will be very less
because of the repulsion existing between the bond pairs. Hence, VSEPR theory also
supports a tetrahedral structure for CH.

© 2026 GoodEd Technologies Pvt. Ltd.