4.23 Which out of NH and NFhas higher dipole moment and why ?
In both molecules i.e., NH and NF, the central atom (N) has a lone pair electron and
there are three bond pairs. Hence, both molecules have a pyramidal shape. Since fluorine
is more electronegative than hydrogen, it is expected that the net dipole moment of NF
is greater than NH. However, the net dipole moment of NH (1.46 D) is greater than that
of NF (0.24 D).
This can be explained on the basis of the directions of the dipole moments of each
individual bond in NF and NH. These directions can be shown as:
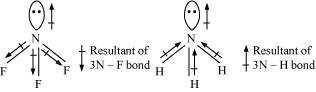
Thus, the resultant moment of the N–H bonds add up to the bond moment of the lone pair
(the two being in the same direction), whereas that of the three N – F bonds partly cancels
the moment of the lone pair.
Hence, the net dipole moment of NF is less than that of NH.

© 2026 GoodEd Technologies Pvt. Ltd.