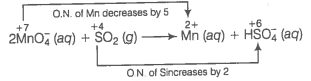
(a) Ion electron method Write the skeleton equation for the given reaction.
Find out the elements which undergo change in O.N.
Divide the given skeleton into two half equations.
Reduction half equation :
Oxidation half equation :
To balance reduction half equation
In acidic medium, balance H and O-atoms
To balance the complete reaction
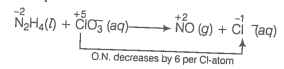
(b) Oxidation number method Write the skeleton equation for the given reaction.
O.N. increases by 4 per N-atom
Multiply NO by 2 because in N2H4 there are 2N atoms
Total increase in O.N. of
Total decrease in O.N. of
Therefore, to balance increase or decrease in O.N. multiply by 3, 2NO by 3 and by 4
Balance O and H-atoms by adding to RHS
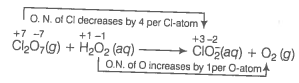
(c) Ion electron method Write the skeleton equation for the given reaction.
Find out the elements which undergo a change in O.N.
Divide the given skeleton equation into two half equations.
Reduction half equation :
Oxidation half equation :
To balance the reduction half equation
To balance the oxidation half equation
To balance the complete reaction
This represents the balanced redox reaction.