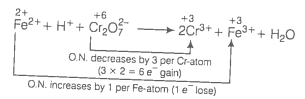
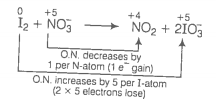
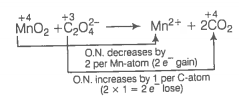
Oxidation number method
(a)
(Multiply Cr3+ by 2 because there are 2Cr atoms in ion.)
Balance increase and decrease in oxidation number.
Balance charge by multiplying H+ by 14.
Balance H and O-atoms by multiplying H2O by 7.
This represents a balanced redox reaction.
(b)
(Multiply by 2 because there are 4 S-atoms in ion.)
Increase and decrease in oxidation number is already balanced. Charge and oxygen atoms are also balanced.
This represents a balanced redox reaction.
(c)
Increase and decrease in oxidation number is already balanced.
Add 4H+ towards LHS of the equation to balance charge.
Add 2H2O towards RHS of the equation to balance H-atoms.
This represents a balanced redox reaction.