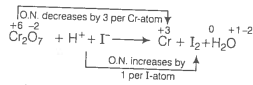
(a) Write the O.N. of all atoms above their respective symbols.
O.N. decreases by, 3 per Cr-atom
Divide the given equation into two half reactions
Reduction half reaction :
Oxidation half reaction :
To balance reduction half reaction.
To balance oxidation half reaction.
To balance reaction by electrons gained and lost
This gives the final balanced ionic equations.
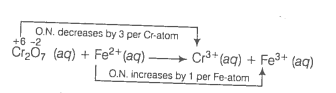
(b) Write the skeletal equation of the given reaction
Write the O.N. of all the elements above their respective symbols.
Divide the given equation into two half reactions
Oxidation half reaction :
Reduction half reaction :
To balance oxidation half reaction
To balance reduction half reaction
Balance charge by adding H+ ions.
Balance O atoms by adding H2O molecules
To balance the reaction
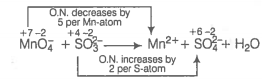
(c) Write the O.N. of all atoms above their respective symbols.
Divide the skeleton equation into two half-reactions.
Reduction half reaction :
Oxidation half reaction :
To balance reduction half reaction
To balance oxidation half reaction
Balance charge by adding H+ ions.
Balance O-atoms by adding H2O
To balance the reaction
This represents the correct balance redox equation.
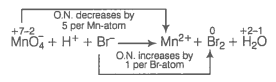
(d) Write the O.N. of all the atoms above their respective symbols.
Divide skeleton equation into two half reactions
Reduction half reaction :
Oxidation half reaction :
To balance half reaction
To balance oxidation half reaction
To balance the reaction
This represents the correct balance ionic equation.