Why is a solution of potassium hydroxide (KOH) used to absorb carbon dioxide (\(CO_2\)) evolved during the estimation of carbon in an organic compound?
| 1. | KOH converts \(CO_2\) into potassium oxide. |
| 2. | KOH reacts with \(CO_2\) to form potassium carbonate, which is measurable. |
| 3. | KOH condenses \(CO_2\), raising the U-tube’s temperature. |
| 4. | KOH absorbs \(CO_2\) and moisture without forming a compound. |
Carbon dioxide is acidic in nature and potassium hydroxide is a strong base. Hence, carbon
dioxide reacts with potassium hydroxide to form potassium carbonate and water as:
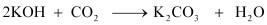
Thus, the mass of the U-tube containing KOH increases. This increase in the mass of Utube
gives the mass of CO2 produced. From its mass, the percentage of carbon in the
organic compound can be estimated.

© 2026 GoodEd Technologies Pvt. Ltd.