Question 12.15:
What is the relationship between the members of following pairs of structures? Are they
structural or geometrical isomers or resonance contributors?
(a) 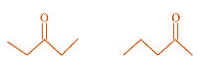
(c) 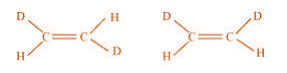
(d) 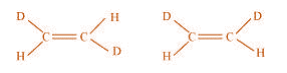
(a) Compounds having the same molecular formula but with different structures are
called structural isomers. The given compounds have the same molecular formula but they
differ in the position of the functional group (ketone group).

In structure I, ketone group is at the C-3 of the parent chain (hexane chain) and in
structure II, ketone group is at the C-2 of the parent chain (hexane chain). Hence, the
given pair represents structural isomers.
(b) Compounds having the same molecular formula, the same constitution, and the
sequence of covalent bonds, but with different relative position of their atoms in space are
called geometrical isomers.
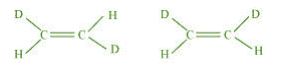
In structures I and II, the relative position of Deuterium (D) and hydrogen (H) in space
are different. Hence, the given pairs represent geometrical isomers.
(c) The given structures are canonical structures or contributing structures. They are
hypothetical and individually do not represent any real molecule. Hence, the given pair
represents resonance structures, called resonance isomers.


© 2026 GoodEd Technologies Pvt. Ltd.