6.22 Out of C and CO, which is a better reducing agent for ZnO ?
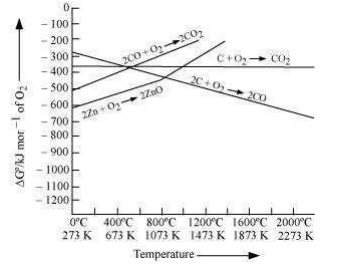
Reduction of ZnO to Zn is usually carried out at 1673 K. From the above figure, it can be observed that above 1073 K, the Gibbs free energy of formation of CO from C and above 1273 K, the Gibbs free energy of formation of from C is lesser than the Gibbs free energy of formation of ZnO. Therefore, C can easily reduce ZnO to Zn. On the other hand, the Gibbs free energy of formation of from CO is always higher than the Gibbs free energy of formation of ZnO. Therefore, CO cannot reduce ZnO.
Hence, C is a better reducing agent than CO for reducing ZnO.

© 2026 GoodEd Technologies Pvt. Ltd.