Describe the shapes of
. Assign the hybridization of boron in these species.
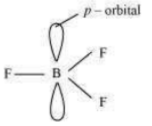
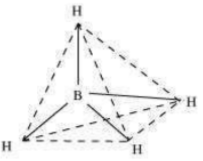
(ii) Boron-hydride ion () is formed by the hybridisation of boron orbitals. Therefore, it is a tetrahedral in structure.

© 2026 GoodEd Technologies Pvt. Ltd.