Suggest reasons why the B–F bond lengths in (130 pm) and (143 pm) differ.
The B–F bond length in is shorter than the B–F bond length in . is an electron-deficient species. With a vacant p-orbital on boron, the fluorine and boron atoms undergo pπ–pπ back-bonding to remove this deficiency. This imparts a double bond character to the B–F bond.
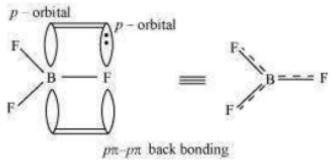
This double-bond character causes the bond length to shorten in (130 pm). However, when coordinates with the fluoride ion, a change in hybridisation from (in ) to
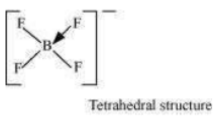
(in ) occurs. Boron now forms 4σ bonds and the double-bond character is lost. This
accounts for a B–F bond length of 143 pm in ion.

© 2026 GoodEd Technologies Pvt. Ltd.