Explain structures of diborane and boric acid.
(a) Diborane
is an electron-deficient compound. has only 12 electrons – 6 from 6 H atoms and 3 each from 2 B atoms. Thus, after combining with 3 H atoms, none of the boron atoms has any electrons left. X-ray diffraction studies have shown the structure of diborane as:
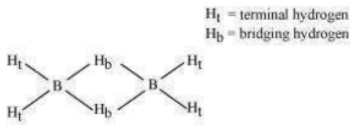
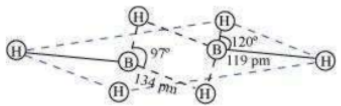
2 boron and 4 terminal hydrogen atoms () lie in one plane, while the other two bridging hydrogen atoms () lie in a plane perpendicular to the plane of boron atoms. Again, of the two bridging hydrogen atoms, one H atom lies above the plane and the other lies below the plane. The terminal bonds are regular two-centre two-electron (2c – 2 ) bonds, while the two bridging (B–H–B) bonds are three-centre two-electron (3c – 2 ) bonds.
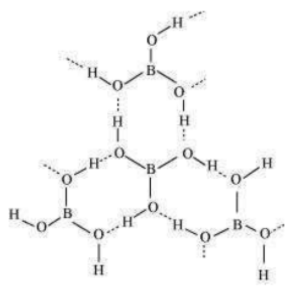
(b) Boric acid
Boric acid has a layered structure. Each planar unit is linked to one another through H atoms. The H atoms form a covalent bond with a unit, while a hydrogen bond is formed with another unit. In the given figure, the dotted lines represent hydrogen bonds.

© 2026 GoodEd Technologies Pvt. Ltd.