Which of the following reactions are disproportionation reactions?
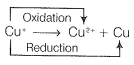
\(\left(\right. i \left.\right) C u^{+} \rightarrow C u^{2 +} + C u\)
\(\left(\right. i i \left.\right) 3 M n O_{4}^{-} + 4 H^{+} \rightarrow 2 M n O_{4}^{-} + M n O_{2} + 2 H_{2} O\)
\(\left(\right. i i i \left.\right) 2 K M n O_{4} \rightarrow K_{2} M n O_{4} + M n O_{2} + O_{2}\)
\(\left(\right. i v \left.\right) 2 M n O_{4}^{-} + 3 M n^{2 +} + 2 H_{2} O \rightarrow 5 M n O_{2} + 4 H^{+}\)
1. (i)
2. (i) , (ii) and (iii)
3. (ii) , (iii) and (iv)
4. (i) and (iv)

© 2026 GoodEd Technologies Pvt. Ltd.