Indicate the complex ion that shows geometrical isomerism:
1. [Cr(H2O)4 Cl2]+
2. [Pt(NH3)3 Cl]
3. [Co(NH3)6]3+
4. [Co(CN)6]3-
shows geometrical isomerism because it is a MAB, type coordination
compound which contains two set of equivalent ligands, four HO and 2 Cl.
Hence, the possible geometrical isomers are
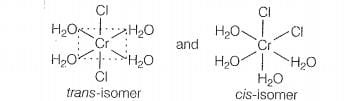
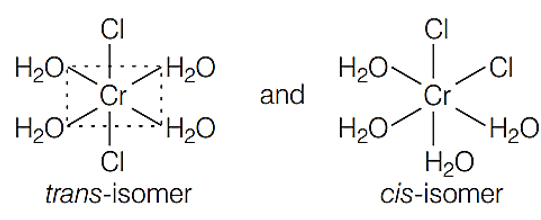
Hence, correct choice is (a).

© 2026 GoodEd Technologies Pvt. Ltd.