Atomic number of Mn Fe and Co are 25, 26 and 27 respectively. Which of the following inner orbital octahedral complex ions are diamagnetic?
Choose the correct option
1. (a, b)
2. (b, c)
3. (c, d)
4. (a, c)
Molecular orbital electronic configuration of CO3+ in
Number of unpaired electron=0
Magnetic property = Diamagnetic
Molecular orbital electronic configuration of
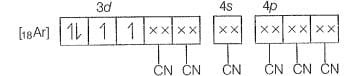
Number of unpaired electron=2
Magnetic property = Paramagnetic
Molecular orbital electronic configuration of is
Number of unpaired electron=2
Magnetic property = Diamagnetic
Molecular orbital electronic configuration of
Number of unpaired electron = 2
Magnetic property = Paramagnetic
Thus,
Hence, correct choices are options (a) and (c).

© 2025 GoodEd Technologies Pvt. Ltd.


