Compound ‘X with molecular formula C,H, Br is treated with aq. KOH
solution. The rate of this reaction depends upon the concentration of
the compound ‘A’ only. When another optically active isomer ’B’ of this
compound was treated with aq. KOH solution, the rate of reaction was
found to be dependent on concentration of compound and KOH both.
1. Write down the structural formula of both compounds ‘A’ and ’B’,
2. Out of these two compounds, which one will be converted to the
product with inverted configuration.
tertiary alky! halide i.e., 2-bromo-2-methylpropane and the structure is as follow
Optically active isomer of (A) is 2-bromobutane (8) and its structural formula is
(ii) As compound (B) is opically active therefore, compound (14) must be 2-bromobutane.
Since. the rate of reaction of compound (B) depends both upon the concentration of
compound (B) and KOH, hence, the reaction follow S2 mechanism. In S2 reaction,
nucleophile attack from, the back side, therefore, the product of hydrolysis will have
‘opposite configuration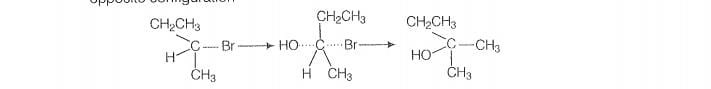

© 2026 GoodEd Technologies Pvt. Ltd.