10.4 Which one of the following has the highest dipole moment?
(i) CH2Cl2
(ii) CHCl3
(iii) CCl4
(i) 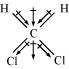
Dichlormethane (CHCl)
μ = 1.60D
(ii) 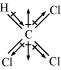
Chloroform (CHCl)
μ = 1.08D
(iii) 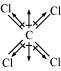
Carbon tetrachloride (CCl)
μ = 0D
CCl is a symmetrical molecule. Therefore, the dipole moments of all four C−Cl bonds
cancel each other. Hence, its resultant dipole moment is zero.
As shown in the above figure, in CHCl, the resultant of dipole moments of two C−Cl bonds
is opposed by the resultant of dipole moments of one C−H bond and one C−Cl bond. Since
the resultant of one C−H bond and one C−Cl bond dipole moments is smaller than two
C−Cl bonds, the opposition is to a small extent. As a result, CHCl has a small dipole
moment of 1.08 D.
On the other hand, in case of CHCl, the resultant of the dipole moments of two C−Cl
bonds is strengthened by the resultant of the dipole moments of two C−H bonds. As a
result, CHCl has a higher dipole moment of 1.60 D than CHCl3 i.e., CHCl has the highest
dipole moment.
Hence, the given compounds can be arranged in the increasing order of their dipole
moments as:
CCl < CHCl < CHCl

© 2026 GoodEd Technologies Pvt. Ltd.