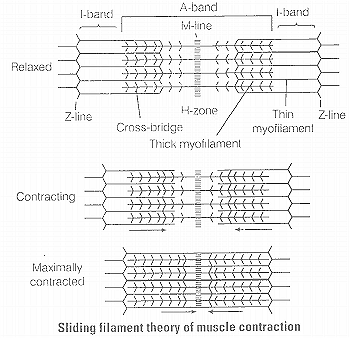
Sliding filament theory
This theory is applicable to smooth, cardiac and skeletal muscles. The essential features of this theory are as follows
(i) During muscle contraction, thin myofilaments slide inward towards the H-zone.
(ii) The sarcomere, the basic unit of muscle contraction, shortens, without changing the length of thin and thick myofilaments.
(iii) The cross-bridge of the thick myofilaments connect with the portions of actin of the thin myofilaments. These cross-bridge move on the surface of the thin myofilaments, resulting in the sliding of thin and thick myofilaments over each other.
(iv) The length of the thick and thin myofilaments do not change during muscle contraction.
(v) A muscle fibre maintains a resting potential under resting conditions just like a nerve fibre. As soon as nerve impulse reaches the terminal end of the axon, small sacs called synaptic vesicles fuse with the axon membrane and release a chemical transmitter, called acetylcholine
It diffuses across the synaptic cleft (the space between the axon membrane and the motor end plate) and binds to the receptor sites of the motor end plate.
(vi) As soon as depolarisation of the motor end plate reaches a certain level. It creates an action potential. Afier this an enzyme cholinesterase present along with the receptor sites for acetylcholine breaks down acetylcholine into acetate and choline.
A portion of the choline diffuses back to the axon and is reused to synthesise more acetylcholine for the transmission of subsequent impulses
(vii) Calcium plays a key regulatory role in muscle contraction. The Ca2+ ions bind to troponin causing a change in its shape and position. This in turn alters the shape and position of tropomyosin.
This shift exposes the active sites on the F-actin molecules and myosin cross-bridges are then able to bind to these active sites.
(viii) The head of each myosin molecule contains an enzyme myosin ATPase. In the presence of myosin ATPase, Ca2+ and Mg2+ ions, ATP breaks down into ADP and inorganic phosphate as
ATP ADP + Pi + Energy
(ix) Energy from ATP causes energised myosin cross-bridges to bind to actin. The energised cross-bridge move, causing the thin myofilaments to slide along the thick myofilaments This movement is like the movement of the oars of a boat.
(x) As stated earlier in theory, there is no shortening of thin and thick myofilaments. However, the sarcomere shortens. because of the sliding of the thin myofilaments produced by cross-bridge movements. The H-zone and I-band shorten. but the width of the A-band remains constant.