It is interesting to learn about those simple experiments that led to a gradual development in our understanding of photosynthesis.
Joseph Priestley (1733-1804) in 1770 performed a series of experiments that revealed the essential role of air in the growth of green plants. Priestley, you may recall, discovered oxygen in 1774. Priestley observed that a candle burning in a closed space – a bell jar, soon gets extinguished (Figure 13.1 a, b, c, d). Similarly, a mouse would soon suffocate in a closed space. He concluded that a burning candle or an animal that breathe the air, both somehow, damage the air. But when he placed a mint plant in the same bell jar, he found that the mouse stayed alive and the candle continued to burn. Priestley hypothesised as follows: Plants restore to the air whatever breathing animals and burning candles remove.
Can you imagine how Priestley would have conducted the experiment using a candle and a plant? Remember, he would need to rekindle the candle to test whether it burns after a few days. How many different ways can you think of to light the candle without disturbing the set-up?
Using a similar setup as the one used by Priestley, but by placing it once in the dark and once in the sunlight, Jan Ingenhousz (1730-1799) showed that sunlight is essential to the plant process that somehow purifies the air fouled by burning candles or breathing animals. Ingenhousz in an elegant experiment with an aquatic plant showed that in bright sunlight, small bubbles were formed around the green parts while in the dark they did not. Later he identified these bubbles to be of oxygen. Hence he showed that it is only the green part of the plants that could release oxygen.
It was not until about 1854 that Julius von Sachs provided evidence for production of glucose when plants grow. Glucose is usually stored as starch. His later studies showed that the green substance in plants (chlorophyll as we know it now) is located in special bodies (later called chloroplasts) within plant cells. He found that the green parts in plants is where glucose is made, and that the glucose is usually stored as starch.
Now consider the interesting experiments done by T.W Engelmann (1843 – 1909). Using a prism he split light into its spectral components and then illuminated a green alga, Cladophora, placed in a suspension of aerobic bacteria. The bacteria were used to detect the sites of O2 evolution. He observed that the bacteria accumulated mainly in the region of blue and red light of the split spectrum. A first action spectrum of photosynthesis was thus described. It resembles roughly the absorption spectra of chlorophyll a and b (discussed in section 13.4).
By the middle of the nineteenth century the key features of plant photosynthesis were known, namely, that plants could use light energy to make carbohydrates from CO2 and water. The empirical equation representing the total process of photosynthesis for oxygen evolving organisms was then understood as:

where [CH2O] represented a carbohydrate (e.g., glucose, a six-carbon sugar).
A milestone contribution to the understanding of photosynthesis was that made by a microbiologist, Cornelius van Niel (1897-1985), who, based on his studies of purple and green bacteria, demonstrated that photosynthesis is essentially a light-dependent reaction in which hydrogen from a suitable oxidisable compound reduces carbon dioxide to carbohydrates. This can be expressed by:

In green plants H2O is the hydrogen donor and is oxidised to O2. Some organisms do not release O2 during photosynthesis. When H2S, instead is the hydrogen donor for purple and green sulphur bacteria, the ‘oxidation’ product is sulphur or sulphate depending on the organism and not O2. Hence, he inferred that the O2 evolved by the green plant comes from H2O, not from carbon dioxide. This was later proved by using radioisotopic techniques. The correct equation, that would represent the overall process of photosynthesis is therefore:
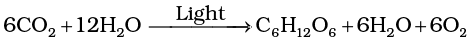
where C6 H12 O6 represents glucose. The O2 released is from water; this was proved using radio isotope techniques. Note that this is not a single reaction but description of a multistep process called photosynthesis. Can you explain why twelve molecules of water as substrate are used in the equation given above?