J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement (Fig. 2.4). Many different names are given to this model, for example, plum pudding, raisin pudding or watermelon. This model can be visualised as a pudding or watermelon of positive charge with plums or seeds (electrons) embedded into it. An important feature of this model is that the mass of the atom is assumed to be uniformly distributed over the atom. Although this model was able to explain the overall neutrality of the atom, but was not consistent with the results of later experiments. Thomson was awarded Nobel Prize for physics in 1906, for his theoretical and experimental investigations on the conduction of electricity by gases.
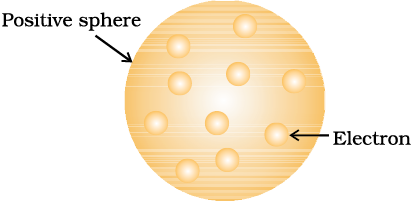
Fig.2.4 Thomson model of atom
In the later half of the nineteenth century different kinds of rays were discovered, besides those mentioned earlier. Wilhalm Röentgen (1845-1923) in 1895 showed that when electrons strike a material in the cathode ray tubes, produce rays which can cause fluorescence in the fluorescent materials placed outside the cathode ray tubes. Since Röentgen did not know the nature of the radiation, he named them X-rays and the name is still carried on. It was noticed that X-rays are produced effectively when electrons strike the dense metal anode, called targets. These are not deflected by the electric and magnetic fields and have a very high penetrating power through the matter and that is the reason that these rays are used to study the interior of the objects. These rays are of very short wavelengths (∼0.1 nm) and possess electro-magnetic character (Section 2.3.1).
Henri Becqueral (1852-1908) observed that there are certain elements which emit radiation on their own and named this phenomenon as radioactivity and the elements known as radioactive elements. This field was developed by Marie Curie, Piere Curie, Rutherford and Fredrick Soddy. It was observed that three kinds of rays i.e., α, β- and γ-rays are emitted. Rutherford found that α-rays consists of high energy particles carrying two units of positive charge and four unit of atomic mass. He concluded that α- particles are helium nuclei as when α- particles combined with two electrons yielded helium gas. β-rays are negatively charged particles similar to electrons. The γ-rays are high energy radiations like X-rays, are neutral in nature and do not consist of particles. As regards penetrating power, α-particles are the least, followed by β-rays (100 times that of α–particles) and γ-rays (1000 times of that α-particles).