In the Lewis description of covalent bond, the Bond Order is given by the number of bonds between the two atoms in a molecule. The bond order, for example in H2 (with a single shared electron pair), in O2 (with two shared electron pairs) and in N2 (with three shared electron pairs) is 1,2,3 respectively. Similarly in CO (three shared electron pairs between C and O) the bond order is 3. For N2, bond order is 3 and its 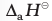 is 946 kJ mol–1; being one of the highest for a diatomic molecule.
is 946 kJ mol–1; being one of the highest for a diatomic molecule.
Isoelectronic molecules and ions have identical bond orders; for example, F2 and O2 2– have bond order 1. N2, CO and NO+ have bond order 3.
A general correlation useful for understanding the stablities of molecules is that: with increase in bond order, bond enthalpy increases and bond length decreases.
It is often observed that a single Lewis structure is inadequate for the representation of a molecule in conformity with its experimentally determined parameters. For example, the ozone, O3 molecule can be equally represented by the structures I and II shown below:
In both structures we have a O–O single bond and a O=O double bond. The normal O–O and O=O bond lengths are 148 pm and 121 pm respectively. Experimentally determined oxygen-oxygen bond lengths in the O3 molecule are same (128 pm). Thus the oxygen-oxygen bonds in the O3 molecule are intermediate between a double and a single bond. Obviously, this cannot be represented by either of the two Lewis structures shown above.
The concept of resonance was introduced to deal with the type of difficulty experienced in the depiction of accurate structures of molecules like O3. According to the concept of resonance, whenever a single Lewis structure cannot describe a molecule accurately, a number of structures with similar energy, positions of nuclei, bonding and non-bonding pairs of electrons are taken as the canonical structures of the hybrid which describes the molecule accurately. Thus for O3, the two structures shown above constitute the canonical structures or resonance structures and their hybrid i.e., the III structure represents the structure of O3 more accurately. This is also called resonance hybrid. Resonance is represented by a double headed arrow.
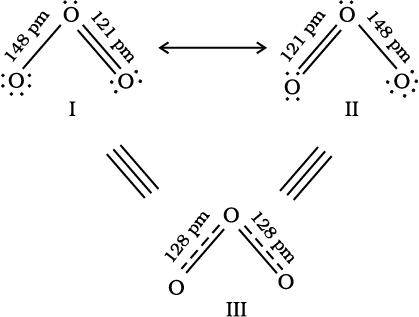
Fig. 4.3 Resonance in the O3 molecule (structures I and II represent the two canonical forms while the structure III is the resonance hybrid)
Some of the other examples of resonance structures are provided by the carbonate ion and the carbon dioxide molecule.
Problem 4.3
Explain the structure of CO32– ion in terms of resonance.
Solution
The single Lewis structure based on the presence of two single bonds and one double bond between carbon and oxygen atoms is inadequate to represent the molecule accurately as it represents unequal bonds. According to the experimental findings, all carbon to oxygen bonds in CO32– are equivalent. Therefore the carbonate ion is best described as a resonance hybrid of the canonical forms I, II, and III shown below.
Fig.4.4 Resonance in CO32–, I, II and III represent the three canonical forms.
Problem 4.4
Explain the structure of CO2 molecule.
Solution
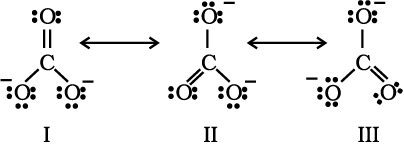
The experimentally determined carbon to oxygen bond length in CO2 is 115 pm. The lengths of a normal carbon to oxygen double bond (C=O) and carbon to oxygen triple bond (C≡O) are 121 pm and 110 pm respectively. The carbon-oxygen bond lengths in CO2 (115 pm) lie between the values for C=O and C≡O. Obviously, a single Lewis structure cannot depict this position and it becomes necessary to write more than one Lewis structures and to consider that the structure of CO2 is best described as a hybrid of the canonical or resonance forms I, II and III.
Fig. 4.5 Resonance in CO2 molecule, I, II and III represent the three canonical forms.
• Resonance stabilizes the molecule as the energy of the resonance hybrid is less than the energy of any single cannonical structure; and,
• Resonance averages the bond characteristics as a whole.
Thus the energy of the O3 resonance hybrid is lower than either of the two cannonical froms I and II (Fig 4.3).
Many misconceptions are associated with resonance and the same need to be dispelled. You should remember that :
• The cannonical forms have no real existence.
• The molecule does not exist for a certain fraction of time in one cannonical form and for other fractions of time in other cannonical forms.
• There is no such equilibrium between the cannonical forms as we have between tautomeric forms (keto and enol) in tautomerism.
• The molecule as such has a single structure which is the resonance hybrid of the cannonical forms and which cannot as such be depicted by a single Lewis structure.