As already explained, Lewis concept is unable to explain the shapes of molecules. This theory provides a simple procedure to predict the shapes of covalent molecules. Sidgwick and Powell in 1940, proposed a simple theory based on the repulsive interactions of the electron pairs in the valence shell of the atoms. It was further developed and redefined by Nyholm and Gillespie (1957).
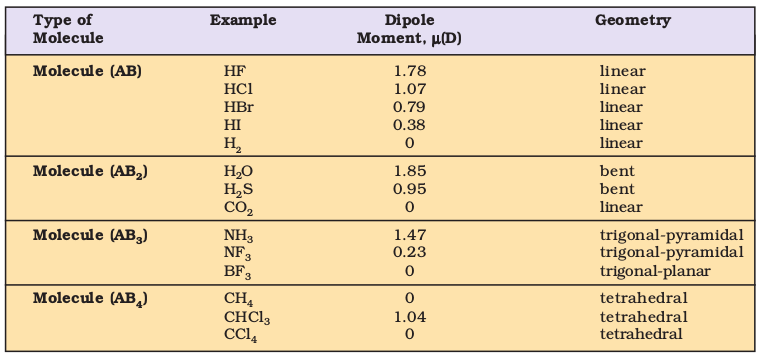
Table 4.5 Dipole Moments of Selected Molecules
The main postulates of VSEPR theory are as follows:
• The shape of a molecule depends upon the number of valence shell electron pairs (bonded or nonbonded) around the central atom.
• Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
• These pairs of electrons tend to occupy such positions in space that minimise repulsion and thus maximise distance between them.
• The valence shell is taken as a sphere with the electron pairs localising on the spherical surface at maximum distance from one another.
• A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair.
• Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structure.
The repulsive interaction of electron pairs decrease in the order:
Lone pair (lp) – Lone pair (lp) > Lone pair (lp) – Bond pair (bp) > Bond pair (bp) – Bond pair (bp)
Nyholm and Gillespie (1957) refined the VSEPR model by explaining the important difference between the lone pairs and bonding pairs of electrons. While the lone pairs are localised on the central atom, each bonded pair is shared between two atoms. As a result, the lone pair electrons in a molecule occupy more space as compared to the bonding pairs of electrons. This results in greater repulsion between lone pairs of electrons as compared to the lone pair - bond pair and bond pair - bond pair repulsions. These repulsion effects result in deviations from idealised shapes and alterations in bond angles in molecules.
For the prediction of geometrical shapes of molecules with the help of VSEPR theory, it is convenient to divide molecules into two categories as (i) molecules in which the central atom has no lone pair and (ii) molecules in which the central atom has one or more lone pairs.
Table 4.6 (page114) shows the arrangement of electron pairs about a central atom A (without any lone pairs) and geometries of some molecules/ions of the type AB. Table 4.7 (page 115) shows shapes of some simple molecules and ions in which the central atom has one or more lone pairs. Table 4.8 (page 116) explains the reasons for the distortions in the geometry of the molecule.
As depicted in Table 4.6, in the compounds of AB2, AB3, AB4, AB5 and AB6, the arrangement of electron pairs and the B atoms around the central atom A are : linear, trigonal planar, tetrahedral, trigonal-bipyramidal and octahedral, respectively. Such arrangement can be seen in the molecules like BF3 (AB3), CH4 (AB4) and PCl5 (AB5) as depicted below by their ball and stick models.
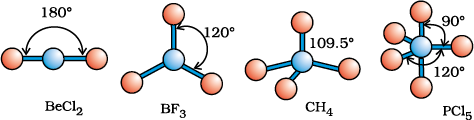
Fig. 4.6 The shapes of molecules in which central atom has no lone pair
The VSEPR Theory is able to predict geometry of a large number of molecules, especially the compounds of p-block elements accurately. It is also quite successful in determining the geometry quite-accurately even when the energy difference between possible structures is very small. The theoretical basis of the VSEPR theory regarding the effects of electron pair repulsions on molecular shapes is not clear and continues to be a subject of doubt and discussion.
Table 4.6 Geometry of Molecules in which the Central Atom has No Lone Pair of Electrons
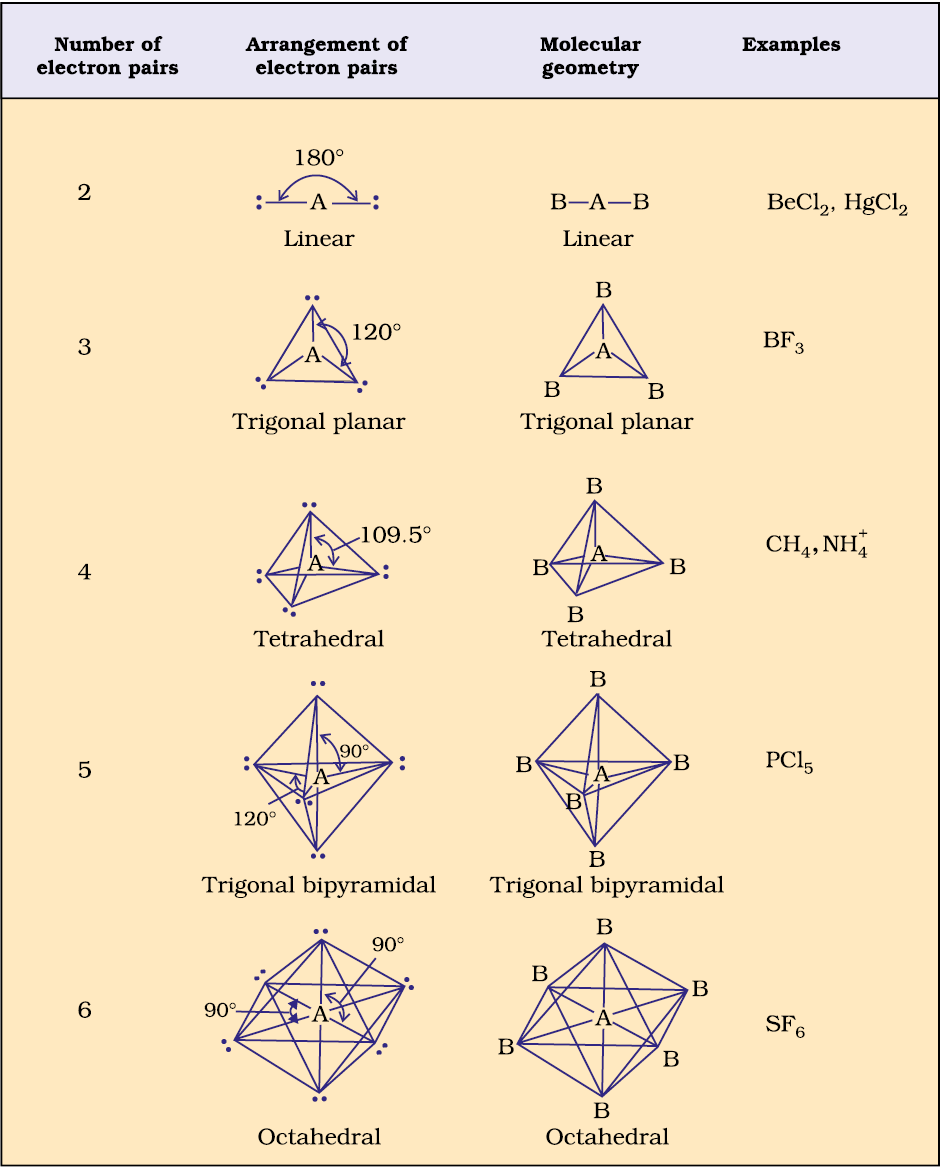
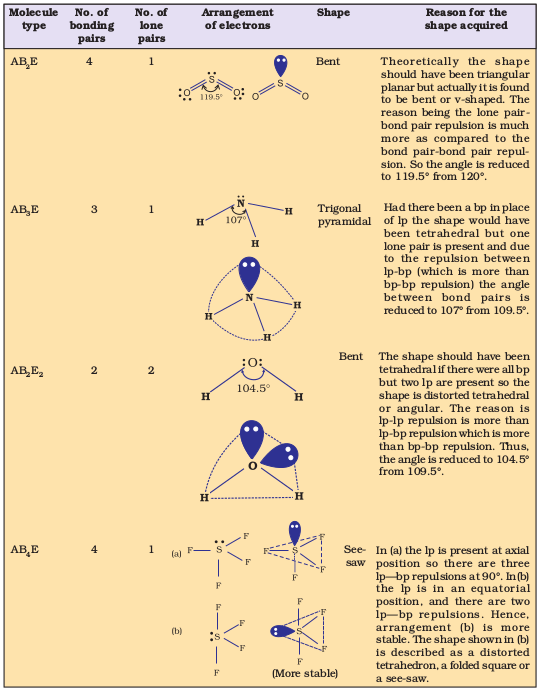
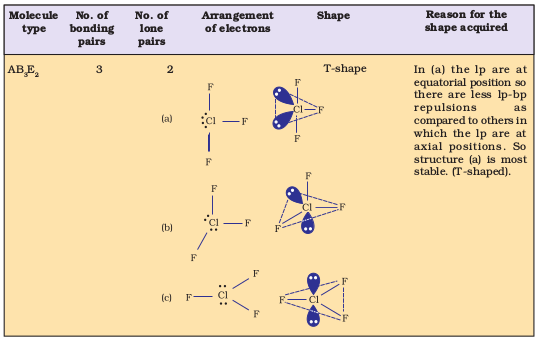
Table 4.7 Shape (geometry) of Some Simple Molecules/Ions with Central Ions having One or More Lone Pairs of Electrons(E).
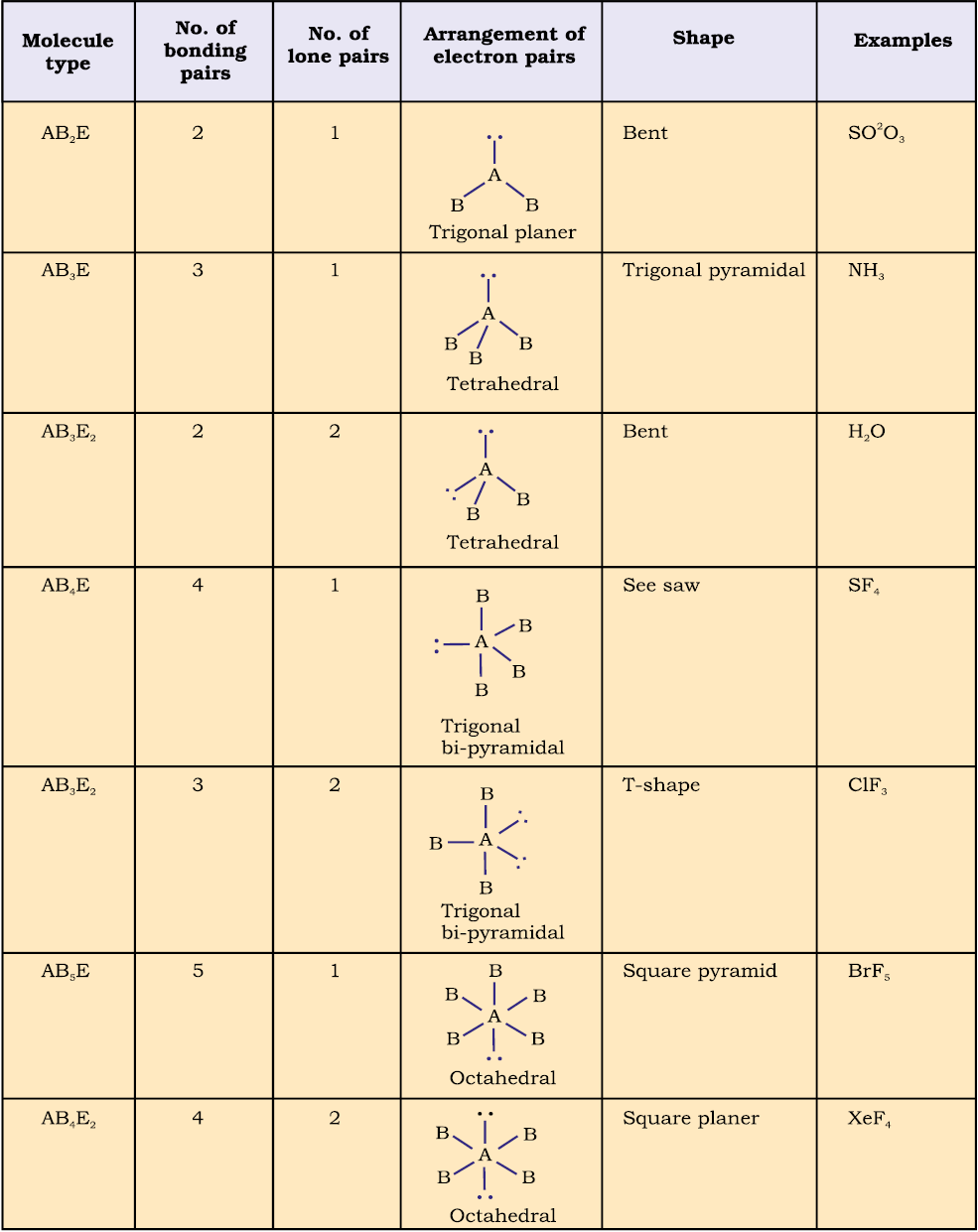
Table 4.8 Shapes of Molecules containing Bond Pair and Lone Pair