In a chemical reaction, reactants are converted into products and is represented by,
Reactants → Products
The enthalpy change accompanying a reaction is called the reaction enthalpy. The enthalpy change of a chemical reaction, is given by the symbol ∆rH
∆rH = (sum of enthalpies of products) – (sum of enthalpies of reactants)
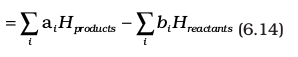
Here symbol Σ (sigma) is used for summation and ai and bi are the stoichiometric coefficients of the products and reactants respectively in the balanced chemical equation. For example, for the reaction
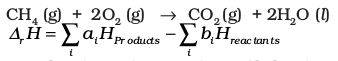
= [Hm (CO2 ,g) + 2Hm (H2O, l)]– [Hm (CH4 , g) + 2Hm (O2, g)]
where Hm is the molar enthalpy.
Enthalpy change is a very useful quantity. Knowledge of this quantity is required when one needs to plan the heating or cooling required to maintain an industrial chemical reaction at constant temperature. It is also required to calculate temperature dependence of equilibrium constant.
(a) Standard Enthalpy of Reactions
Enthalpy of a reaction depends on the conditions under which a reaction is carried out. It is, therefore, necessary that we must specify some standard conditions. The standard enthalpy of reaction is the enthalpy change for a reaction when all the participating substances are in their standard states.
The standard state of a substance at a specified temperature is its pure form at 1 bar. For example, the standard state of liquid ethanol at 298 K is pure liquid ethanol at 1 bar; standard state of solid iron at 500 K is pure iron at 1 bar. Usually data are taken
at 298 K.
Standard conditions are denoted by adding the superscript 0 to the symbol ∆H, e.g., ∆H⊖
(b) Enthalpy Changes during Phase Transformations
Phase transformations also involve energy changes. Ice, for example, requires heat for melting. Normally this melting takes place at constant pressure (atmospheric pressure) and during phase change, temperature remains constant (at 273 K).
H2O(s) → H2O(l); ∆fusH⊖= 6.00 kJ moI–1
Here ∆fusH⊖ is enthalpy of fusion in standard state. If water freezes, then process is reversed and equal amount of heat is given off to the surroundings.
The enthalpy change that accompanies melting of one mole of a solid substance in standard state is called standard enthalpy of fusion or molar enthalpy of fusion, ∆fusH⊖.
Melting of a solid is endothermic, so all enthalpies of fusion are positive. Water requires heat for evaporation. At constant temperature of its boiling point Tb and at constant pressure:
Table 6.1 Standard Enthalpy Changes of Fusion and Vaporisation
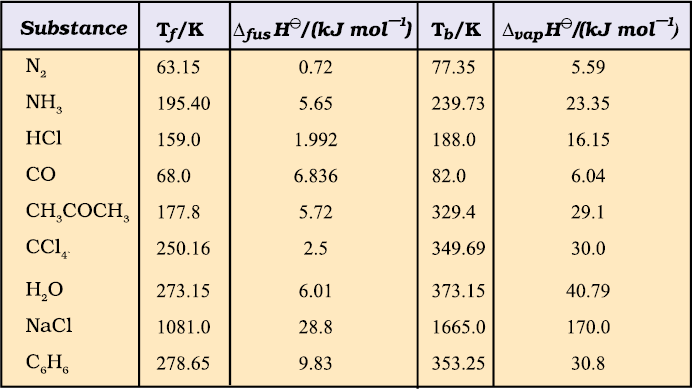
(Tf and Tb are melting and boiling points, respectively)
H2O(l) → H2O(g); ∆vapH⊖= + 40.79 kJ moI–1 ∆vapH⊖is the standard enthalpy of vaporisation.
Amount of heat required to vaporize one mole of a liquid at constant temperature and under standard pressure (1bar) is called its standard enthalpy of vaporization or molar enthalpy of vaporization, ∆vapH⊖.
Sublimation is direct conversion of a solid into its vapour. Solid CO2 or ‘dry ice’ sublimes at 195K with ∆subH⊖=25.2 kJ mol–1; naphthalene sublimes slowly and for this ∆sub H⊖ = 73.0 kJ mol–1 .
Standard enthalpy of sublimation, ∆subH⊖ is the change in enthalpy when one mole of a solid substance sublimes at a constant temperature and under standard pressure (1bar).
The magnitude of the enthalpy change depends on the strength of the intermolecular interactions in the substance undergoing the phase transfomations. For example, the strong hydrogen bonds between water molecules hold them tightly in liquid phase. For an organic liquid, such as acetone, the intermolecular dipole-dipole interactions are significantly weaker. Thus, it requires less heat to vaporise 1 mol of acetone than it does to vaporize 1 mol of water. Table 6.1 gives values of standard enthalpy changes of fusion and vaporisation for some substances.
Problem 6.7
A swimmer coming out from a pool is covered with a film of water weighing about 18g. How much heat must be supplied to evaporate this water at
298 K ? Calculate the internal energy of vaporisation at 298K.
∆vap H⊖ for water
at 298K= 44.01kJ mol–1
Solution
We can represent the process of evaporation as

Table 6.2 Standard Molar Enthalpies of Formation (∆f H) at 298K of a Few Selected Substances
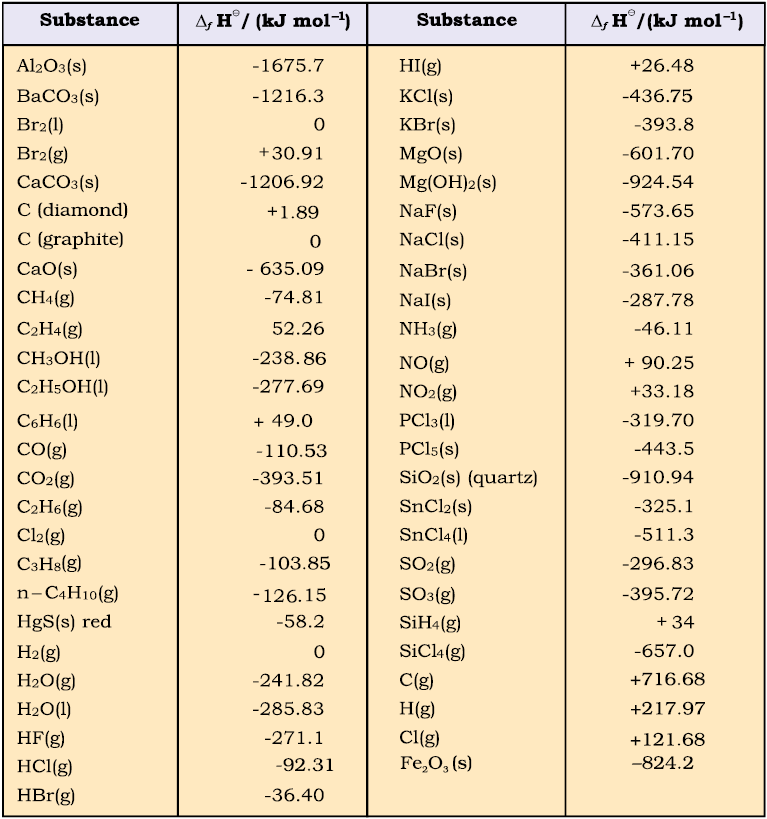
(c) Standard Enthalpy of Formation
The standard enthalpy change for the formation of one mole of a compound from its elements in their most stable states of aggregation (also known as reference states) is called Standard Molar Enthalpy of Formation. Its symbol is ∆fH0, where the subscript ‘ f ’ indicates that one mole of the compound in question has been formed in its standard state from its elements in their most stable states of aggregation. The reference state of an element is its most stable state of aggregation at 25°C and 1 bar pressure.
For example, the reference state of dihydrogen is H2 gas and those of dioxygen, carbon and sulphur are O2 gas, Cgraphite and Srhombic respectively. Some reactions with standard molar enthalpies of formation are as follows.
H2(g) + ½O2 (g) → H2O(1);
∆f H⊖ = –285.8 kJ mol–1
C (graphite, s) + 2H2(g) → Ch4 (g);
∆f H⊖ = –74.81 kJ mol–1
2C (graphite, s)+3H2 (g)+ ½O2(g) → C2H5OH(1);
∆f H⊖ = – 277.7kJ mol–1
It is important to understand that a standard molar enthalpy of formation, ∆fH⊖, is just a special case of ∆rH⊖, where one mole of a compound is formed from its constituent elements, as in the above three equations, where 1 mol of each, water, methane and ethanol is formed. In contrast, the enthalpy change for an exothermic reaction:
CaO(s) + CO2(g) → CaCo3(s);
∆rH⊖= – 178.3kJ mol–1
is not an enthalpy of formation of calcium carbonate, since calcium carbonate has been formed from other compounds, and not from its constituent elements. Also, for the reaction given below, enthalpy change is not standard enthalpy of formation, ∆fH⊖ for HBr(g).
H2(g) + Br2(l) → 2HBr(g);
∆r H⊖= – 178.3kJ mol–1
Here two moles, instead of one mole of the product is formed from the elements, i.e., .
∆r H⊖= 2∆f H⊖
Therefore, by dividing all coefficients in the balanced equation by 2, expression for enthalpy of formation of HBr (g) is written as
½H2(g) + ½Br2(1) → HBr(g);
∆f H⊖= – 36.4 kJ mol–1
Standard enthalpies of formation of some common substances are given in Table 6.2.
By convention, standard enthalpy for formation, ∆fH⊖, of an element in reference state, i.e., its most stable state of aggregation is taken as zero.
Suppose, you are a chemical engineer and want to know how much heat is required to decompose calcium carbonate to lime and carbon dioxide, with all the substances in their standard state.
CaCO3(s) → CaO(s) + CO2(g); ∆r H⊖ = ?
Here, we can make use of standard enthalpy of formation and calculate the enthalpy change for the reaction. The following general equation can be used for the enthalpy change calculation.
∆rH⊖= 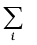 ai ∆f H⊖(products) –
ai ∆f H⊖(products) – 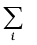 bi ∆f H⊖(reactants) (6.15)
bi ∆f H⊖(reactants) (6.15)
where a and b represent the coefficients of the products and reactants in the balanced equation. Let us apply the above equation for decomposition of calcium carbonate. Here, coefficients ‘a’ and ‘b’ are 1 each. Therefore,
∆rH⊖= ∆f H⊖= [CaO(s)]+ ∆f H⊖[CO2(g)]
– ∆f H⊖= [CaCO3(s)]
=1 (–635.1 kJ mol–1) + 1(–393.5 kJ mol–1)
–1(–1206.9 kJ mol–1)
= 178.3 kJ mol–1
Thus, the decomposition of CaCO3 (s) is an endothermic process and you have to heat it for getting the desired products.
(d) Thermochemical Equations
A balanced chemical equation together with the value of its ∆rH is called a thermochemical equation. We specify the physical state (alongwith allotropic state) of the substance in an equation. For example:
C2H5OH(l) + 3O2(g) → 2CO2(g) + 3H2O(l);
∆rH⊖= – 1367 kJ mol–1
The above equation describes the combustion of liquid ethanol at constant temperature and pressure. The negative sign of enthalpy change indicates that this is an exothermic reaction.
It would be necessary to remember the following conventions regarding thermo-chemical equations.
1. The coefficients in a balanced thermo-chemical equation refer to the number of moles (never molecules) of reactants and products involved in the reaction.
2. The numerical value of ∆rH⊖ refers to the number of moles of substances specified by an equation. Standard enthalpy change ∆rH⊖ will have units as kJ mol–1.
To illustrate the concept, let us consider the calculation of heat of reaction for the following reaction :
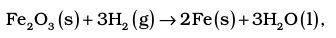
From the Table (6.2) of standard enthalpy of formation (∆f H⊖), we find :
∆f H⊖ (H2O,l) = –285.83 kJ mol–1;
∆f H⊖ (Fe2O3,s) = – 824.2 kJ mol–1;
Also ∆f H⊖ (Fe, s) = 0 and
∆f H⊖ (H2, g) = 0 as per convention
Then,
∆f H1⊖ = 3(–285.83 kJ mol–1)
– 1(– 824.2 kJ mol–1)
= (–857.5 + 824.2) kJ mol–1
= –33.3 kJ mol–1
Note that the coefficients used in these calculations are pure numbers, which are equal to the respective stoichiometric coefficients. The unit for ∆rH⊖ is kJ mol–1, which means per mole of reaction. Once we balance the chemical equation in a particular way, as above, this defines the mole of reaction. If we had balanced the equation differently, for example,
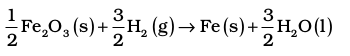
then this amount of reaction would be one mole of reaction and ∆rH⊖ would be
∆f H2⊖ = 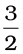 (–285.83 kJ mol–1)
(–285.83 kJ mol–1)
– 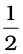 (–824.2 kJ mol–1)
(–824.2 kJ mol–1)
= (– 428.7 + 412.1) kJ mol–1
= –16.6 kJ mol–1 = ½ ∆r H1⊖
It shows that enthalpy is an extensive quantity.
3. When a chemical equation is reversed, the value of ∆rH⊖is reversed in sign. For example
N2(g) + 3H2 (g) → 2NH3 (g);
∆r H⊖= – 91.8 kJ. mol–1
2NH3(g) → N2(g) + 3H2 (g);
∆r H⊖= + 91.8 kJ mol–1
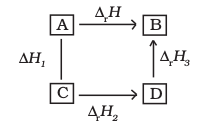
(e) Hess’s Law of Constant Heat Summation
We know that enthalpy is a state function, therefore the change in enthalpy is independent of the path between initial state (reactants) and final state (products). In other words, enthalpy change for a reaction is the same whether it occurs in one step or in a series of steps. This may be stated as follows in the form of Hess’s Law.
If a reaction takes place in several steps then its standard reaction enthalpy is the sum of the standard enthalpies of the intermediate reactions into which the overall reaction may be divided at the same temperature.
Let us understand the importance of this law with the help of an example.
Consider the enthalpy change for the reaction
C (graphite,s) + 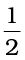 O2 (g) → CO (g); ∆r H⊖= ?
O2 (g) → CO (g); ∆r H⊖= ?
Although CO(g) is the major product, some CO2 gas is always produced in this reaction. Therefore, we cannot measure enthalpy change for the above reaction directly. However, if we can find some other reactions involving related species, it is possible to calculate the enthalpy change for the above reaction.
Let us consider the following reactions:
C (graphite,s) + O2 (g) → CO2 (g);
∆r H⊖= – 393.5 kJ mol–1 (i)
CO (g) + 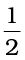 O2 (g) → CO2 (g)
O2 (g) → CO2 (g)
∆r H⊖= – 283.0 kJ mol–1 (ii)
We can combine the above two reactions in such a way so as to obtain the desired reaction. To get one mole of CO(g) on the right, we reverse equation (ii). In this, heat is absorbed instead of being released, so we change sign of ∆rH⊖ value
CO2 (g) → CO (g) + 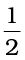 O2 (g);
O2 (g);
∆r H⊖= + 283.0 kJ mol–1 (iii)
Adding equation (i) and (iii), we get the desired equation,
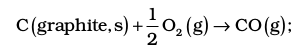
for which ∆r H⊖ = (– 393.5 + 283.0)
= – 110.5 kJ mol–1
In general, if enthalpy of an overall reaction A→B along one route is ∆rH and ∆rH1, ∆rH2, ∆rH3..... representing enthalpies of reactions leading to same product, B along another route,then we have
∆rH = ∆rH1 + ∆rH2 + ∆rH3 ... (6.16)
It can be represented as: