The first law of thermodynamics tells us about the relationship between the heat absorbed and the work performed on or by a system. It puts no restrictions on the direction of heat flow. However, the flow of heat is unidirectional from higher temperature to lower temperature. In fact, all naturally occurring processes whether chemical or physical will tend to proceed spontaneously in one direction only. For example, a gas expanding to fill the available volume, burning carbon in dioxygen giving carbon dioxide.
But heat will not flow from colder body to warmer body on its own, the gas in a container will not spontaneously contract into one corner or carbon dioxide will not form carbon and dioxygen spontaneously. These and many other spontaneously occurring changes show unidirectional change. We may ask ‘what is the driving force of spontaneously occurring changes ? What determines the direction of a spontaneous change ? In this section, we shall establish some criterion for these processes whether these will take place or not.
Let us first understand what do we mean by spontaneous reaction or change ? You may think by your common observation that spontaneous reaction is one which occurs immediately when contact is made between the reactants. Take the case of combination of hydrogen and oxygen. These gases may be mixed at room temperature and left for many years without observing any perceptible change. Although the reaction is taking place between them, it is at an extremely slow rate. It is still called spontaneous reaction. So spontaneity means ‘having the potential to proceed without the assistance of external agency’. However, it does not tell about the rate of the reaction or process. Another aspect of spontaneous reaction or process, as we see is that these cannot reverse their direction on their own. We may summarise it as follows:
A spontaneous process is an irreversible process and may only be reversed by some external agency.
(a) Is Decrease in Enthalpy a Criterion for Spontaneity ?
If we examine the phenomenon like flow of water down hill or fall of a stone on to the ground, we find that there is a net decrease in potential energy in the direction of change. By analogy, we may be tempted to state that a chemical reaction is spontaneous in a given direction, because decrease in energy has taken place, as in the case of exothermic reactions. For example:
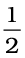 N2(g) +
N2(g) + 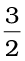 H2(g) = NH3(g) ;
H2(g) = NH3(g) ;
∆r H⊖ = – 46.1 kJ mol–1
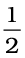 H2(g) +
H2(g) + 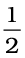 Cl2(g) = HCl (g) ;
Cl2(g) = HCl (g) ;
∆r H⊖ = – 92.32 kJ mol–1
H2(g) + 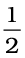 O2(g) → H2O(l) ;
O2(g) → H2O(l) ;
∆r H⊖ = –285.8 kJ mol–1
The decrease in enthalpy in passing from reactants to products may be shown for any exothermic reaction on an enthalpy diagram as shown in Fig. 6.10(a).
Thus, the postulate that driving force for a chemical reaction may be due to decrease in energy sounds ‘reasonable’ as the basis of evidence so far !
Now let us examine the following reactions:
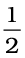 N2(g) + O2(g) → NO2(g);
N2(g) + O2(g) → NO2(g);
∆r H⊖ = +33.2 kJ mol–1
C(graphite, s) + 2 S(l) → CS2(l);
∆r H⊖ = +128.5 kJ mol–1
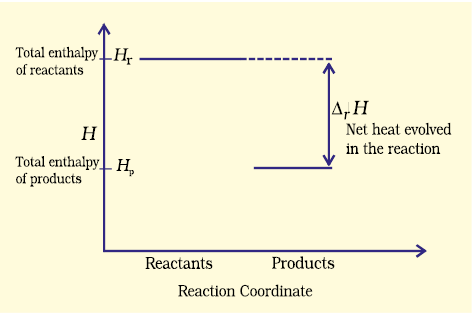
Fig. 6.10 (a) Enthalpy diagram for exothermic reactions
These reactions though endothermic, are spontaneous. The increase in enthalpy may be represented on an enthalpy diagram as shown in Fig. 6.10(b).
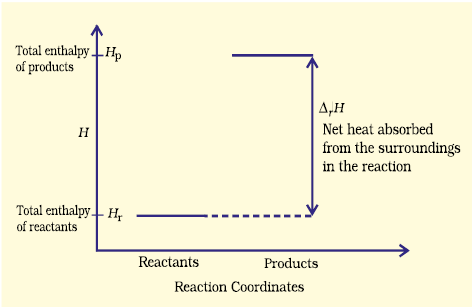
Fig. 6.10 (b) Enthalpy diagram for endothermic reactions
Therefore, it becomes obvious that while decrease in enthalpy may be a contributory factor for spontaneity, but it is not true for all cases.
(b) Entropy and Spontaneity
Then, what drives the spontaneous process in a given direction ? Let us examine such a case in which ∆H = 0 i.e., there is no change in enthalpy, but still the process is spontaneous.
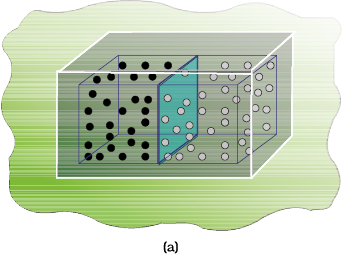
Let us consider diffusion of two gases into each other in a closed container which is isolated from the surroundings as shown in Fig. 6.11.
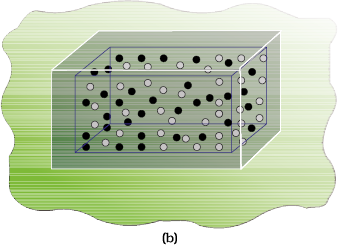
The two gases, say, gas A and gas B are represented by black dots and white dots respectively and separated by a movable partition [Fig. 6.11 (a)]. When the partition is withdrawn [Fig.6.11( b)], the gases begin to diffuse into each other and after a period of time, diffusion will be complete.
Let us examine the process. Before partition, if we were to pick up the gas molecules from left container, we would be sure that these will be molecules of gas A and similarly if we were to pick up the gas molecules from right container, we would be sure that these will be molecules of gas B. But, if we were to pick up molecules from container when partition is removed, we are not sure whether the molecules picked are of gas A or gas B. We say that the system has become less predictable or more chaotic.
We may now formulate another postulate: in an isolated system, there is always a tendency for the systems’ energy to become more disordered or chaotic and this could be a criterion for spontaneous change !
Fig. 6.11 Diffusion of two gases
At this point, we introduce another thermodynamic function, entropy denoted as S. The above mentioned disorder is the manifestation of entropy. To form a mental picture, one can think of entropy as a measure of the degree of randomness or disorder in the system. The greater the disorder in an isolated system, the higher is the entropy. As far as a chemical reaction is concerned, this entropy change can be attributed to rearrangement of atoms or ions from one pattern in the reactants to another (in the products). If the structure of the products is very much disordered than that of the reactants, there will be a resultant increase in entropy. The change in entropy accompanying a chemical reaction may be estimated qualitatively by a consideration of the structures of the species taking part in the reaction. Decrease of regularity in structure would mean increase in entropy. For a given substance, the crystalline solid state is the state of lowest entropy (most ordered), The gaseous state is state of highest entropy.
Now let us try to quantify entropy. One way to calculate the degree of disorder or chaotic distribution of energy among molecules would be through statistical method which is beyond the scope of this treatment. Other way would be to relate this process to the heat involved in a process which would make entropy a thermodynamic concept. Entropy, like any other thermodynamic property such as internal energy U and enthalpy H is a state function and ∆S is independent of path.
Whenever heat is added to the system, it increases molecular motions causing increased randomness in the system. Thus heat (q) has randomising influence on the system. Can we then equate ∆S with q ? Wait ! Experience suggests us that the distribution of heat also depends on the temperature at which heat is added to the system. A system at higher temperature has greater randomness in it than one at lower temperature. Thus, temperature is the measure of average chaotic motion of particles in the system. Heat added to a system at lower temperature causes greater randomness than when the same quantity of heat is added to it at higher temperature. This suggests that the entropy change is inversely proportional to the temperature. ∆S is related with q and T for a reversible reaction as :

The total entropy change ( ∆Stotal) for the system and surroundings of a spontaneous process is given by

When a system is in equilibrium, the entropy is maximum, and the change in entropy, ∆S = 0.
We can say that entropy for a spontaneous process increases till it reaches maximum and at equilibrium the change in entropy is zero. Since entropy is a state property, we can calculate the change in entropy of a reversible process by
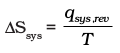
We find that both for reversible and irreversible expansion for an ideal gas, under isothermal conditions, ∆U = 0, but ∆Stotal i.e., ∆Ssys + ∆surr is not zero for irreversible process. Thus, ∆U does not discriminate between reversible and irreversible process, whereas ∆S does.
Problem 6.9
(i) A liquid crystallizes into a solid.
(ii) Temperature of a crystalline solid is raised from 0 K to 115 K.
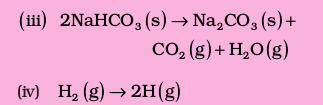
Solution
(i) After freezing, the molecules attain an ordered state and therefore, entropy decreases.
(ii) At 0 K, the contituent particles are static and entropy is minimum. If temperature is raised to 115 K, these begin to move and oscillate about their equilibrium positions in the lattice and system becomes more disordered, therefore entropy increases.
(iii) Reactant, NaHCO3 is a solid and it has low entropy. Among products there are one solid and two gases. Therefore, the products represent a condition of higher entropy.
Problem 6.10
For oxidation of iron,
entropy change is – 549.4 JK–1 mol–1 at 298 K. Inspite of negative entropy change
of this reaction, why is the reaction spontaneous? ( for this reaction is –1648 × 103 J mol –1)
Solution
One decides the spontaneity of a reaction by considering
. For calculating , we have to consider the heat
absorbed by the surroundings which is equal to . At temperature T, entropy
change of the surroundings is
Thus, total entropy change for this reaction
This shows that the above reaction is spontaneous.
(c) Gibbs Energy and Spontaneity
We have seen that for a system, it is the total entropy change, ∆Stotal which decides the spontaneity of the process. But most of the chemical reactions fall into the category of either closed systems or open systems. Therefore, for most of the chemical reactions there are changes in both enthalpy and entropy. It is clear from the discussion in previous sections that neither decrease in enthalpy nor increase in entropy alone can determine the direction of spontaneous change for these systems.
For this purpose, we define a new thermodynamic function the Gibbs energy or Gibbs function, G, as
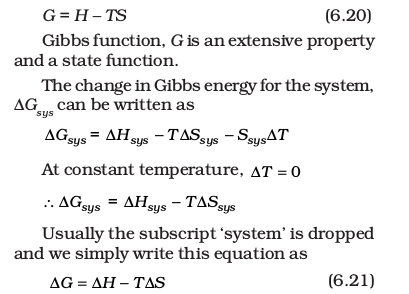
Thus, Gibbs energy change = enthalpy change – temperature × entropy change, and is referred to as the Gibbs equation, one of the most important equations in chemistry. Here, we have considered both terms together for spontaneity: energy (in terms of ∆H) and entropy (∆S, a measure of disorder) as indicated earlier. Dimensionally if we analyse, we find that ∆G has units of energy because, both ∆H and the T∆S are energy terms, since T∆S = (K) (J/K) = J.
Now let us consider how ∆G is related to reaction spontaneity.
We know,
∆Stotal = ∆Ssys + ∆Ssurr
If the system is in thermal equilibrium with the surrounding, then the temperature of the surrounding is same as that of the system. Also, increase in enthalpy of the surrounding is equal to decrease in the enthalpy of the system.
Therefore, entropy change of surroundings,
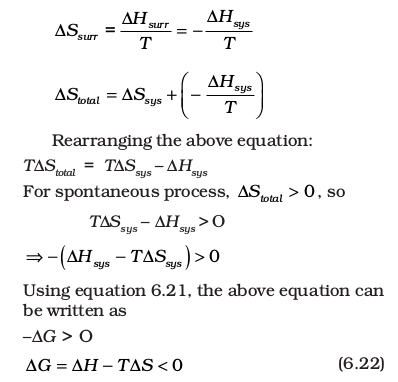
∆Hsys is the enthalpy change of a reaction, T∆Ssys is the energy which is not available to do useful work. So ∆G is the net energy available to do useful work and is thus a measure of the ‘free energy’. For this reason, it is also known as the free energy of the reaction.
∆G gives a criteria for spontaneity at constant pressure and temperature.
(i) If ∆G is negative (< 0), the process is spontaneous.
(ii) If ∆G is positive (> 0), the process is non spontaneous.
Note : If a reaction has a positive enthalpy change and positive entropy change, it can be spontaneous when T∆S is large enough to outweigh ∆H. This can happen in two ways; (a) The positive entropy change of the system can be ‘small’ in which case T must be large. (b) The positive entropy change of the system can be ’large’, in which case T may be small. The former is one of the reasons why reactions are often carried out at high temperature. Table 6.4 summarises the effect of temperature on spontaneity of reactions.
(d) Entropy and Second Law of Thermodynamics
We know that for an isolated system the change in energy remains constant. Therefore, increase in entropy in such systems is the natural direction of a spontaneous change. This, in fact is the second law of thermodynamics. Like first law of thermodynamics, second law can also be stated in several ways. The second law of thermodynamics explains why spontaneous exothermic reactions are so common. In exothermic reactions heat released by the reaction increases the disorder of the surroundings and overall entropy change is positive which makes the reaction spontaneous.
(e) Absolute Entropy and Third Law of Thermodynamics
Molecules of a substance may move in a straight line in any direction, they may spin like a top and the bonds in the molecules may stretch and compress. These motions of the molecule are called translational, rotational and vibrational motion respectively. When temperature of the system rises, these motions become more vigorous and entropy increases. On the other hand when temperature is lowered, the entropy decreases. The entropy of any pure crystalline substance approaches zero as the temperature approaches absolute zero. This is called third law of thermodynamics. This is so because there is perfect order in a crystal at absolute zero. The statement is confined to pure crystalline solids because theoretical arguments and practical evidences have shown that entropy of solutions and super cooled liquids is not zero at 0 K. The importance of the third law lies in the fact that it permits the calculation of absolute values of entropy of pure substance from thermal data alone. For a pure substance, this can be done by summing 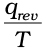 increments from 0 K to 298 K. Standard entropies can be used to calculate standard entropy changes by a Hess’s law type of calculation.
increments from 0 K to 298 K. Standard entropies can be used to calculate standard entropy changes by a Hess’s law type of calculation.