It is due to the presence of soluble salts of magnesium and calcium in the form of chlorides and sulphates in water. Permanent hardness is not removed by boiling. It can be removed by the following methods:
(i) Treatment with washing soda (sodium carbonate): Washing soda reacts with soluble calcium and magnesium chlorides and sulphates in hard water to form insoluble carbonates.
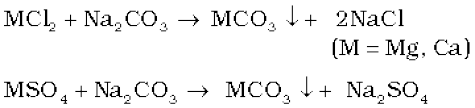
(ii) Calgon’s method: Sodium hexameta-phosphate (Na6P6O18), commercially called ‘calgon’, when added to hard water, the following reactions take place.
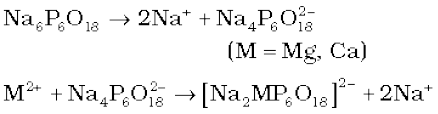
The complex anion keeps the Mg2+ and Ca2+ ions in solution.
(iii) Ion-exchange method: This method is also called zeolite/permutit process. Hydrated sodium aluminium silicate is zeolite/permutit. For the sake of simplicity, sodium aluminium silicate (NaAlSiO4) can be written as NaZ. When this is added in hard water, exchange reactions take place.
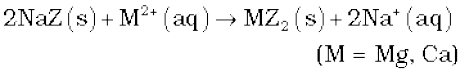
Permutit/zeolite is said to be exhausted when all the sodium in it is used up. It is regenerated for further use by treating with an aqueous sodium chloride solution.
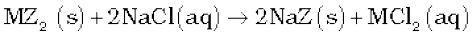
(iv) Synthetic resins method: Nowadays hard water is softened by using synthetic cation exchangers. This method is more efficient than zeolite process. Cation exchange resins contain large organic molecule with - SO3H group and are water insoluble. Ion exchange resin (RSO3H) is changed to RNa by treating it with NaCl. The resin exchanges Na+ ions with Ca2+ and Mg2+ ions present in hard water to make the water soft. Here R is resin anion.
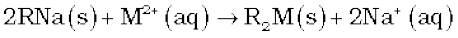
The resin can be regenerated by adding aqueous NaCl solution.
Pure de-mineralised (de-ionized) water free from all soluble mineral salts is obtained by passing water successively through a cation exchange (in the H+ form) and an anion-exchange (in the OH– form) resins:

In this cation exchange process, H+ exchanges for Na+, Ca2+, Mg2+ and other cations present in water. This process results in proton release and thus makes the water acidic. In the anion exchange process:
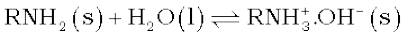
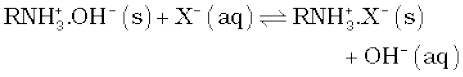
OH–exchanges for anions like Cl–, HCO3–, SO42–etc. present in water. OH– ions, thus, liberated neutralise the H+ ions set free in the cation exchange.
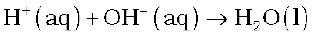
The exhausted cation and anion exchange resin beds are regenerated by treatment with dilute acid and alkali solutions respectively.