All the alkali metals are silvery white, soft and light metals. Because of the large size, these elements have low density which increases down the group from Li to Cs. However, potassium is lighter than sodium. The melting and boiling points of the alkali metals are low indicating weak metallic bonding due to the presence of only a single valence electron in them. The alkali metals and their salts impart characteristic colour to an oxidizing flame. This is because the heat from the flame excites the outermost orbital electron to a higher energy level. When the excited electron comes back to the ground state, there is emission of radiation in the visible region of the spectrum as given below:
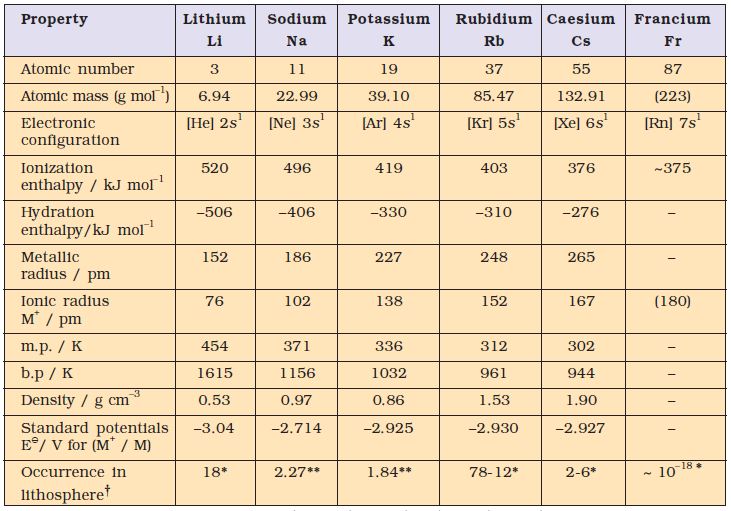
*ppm (part per million), ** percentage by weight; † Lithosphere: The Earth’s outer layer: its crust and part of the upper mantle
The alkali metals are highly reactive due to their large size and low ionization enthalpy. The reactivity of these metals increases down the group.
(i) Reactivity towards air: The alkali metals tarnish in dry air due to the formation of their oxides which in turn react with moisture to form hydroxides. They burn vigorously in oxygen forming oxides. Lithium forms monoxide, sodium forms peroxide, the other metals form superoxides. The superoxide O2– ion is stable only in the presence of large cations such as K, Rb, Cs.
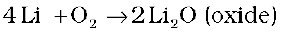
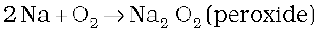
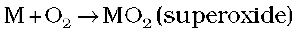
(M = K, Rb, Cs)
In all these oxides the oxidation state of the alkali metal is +1. Lithium shows exceptional behaviour in reacting directly with nitrogen of air to form the nitride, Li3N as well. Because of their high reactivity towards air and water, alkali metals are normally kept in kerosene oil.
Problem 10.1
What is the oxidation state of K in KO2?
Solution
The superoxide species is represented as O2–; since the compound is neutral, therefore, the oxidation state of potassium
is +1.
(ii) Reactivity towards water: The alkali metals react with water to form hydroxide and dihydrogen.
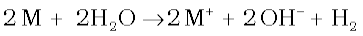
(M = an alkali metal)
It may be noted that although lithium has most negative E value (Table 10.1), its reaction with water is less vigorous than that of sodium which has the least negative E value among the alkali metals. This behaviour of lithium is attributed to its small size and very high hydration energy. Other metals of the group react explosively with water.
They also react with proton donors such as alcohol, gaseous ammonia and alkynes.
(iii) Reactivity towards dihydrogen: The alkali metals react with dihydrogen at about 673K (lithium at 1073K) to form hydrides. All the alkali metal hydrides are ionic solids with high melting points.
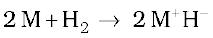
(iv) Reactivity towards halogens: The alkali metals readily react vigorously with halogens to form ionic halides, M+X–. However, lithium halides are somewhat covalent. It is because of the high polarisation capability of lithium ion (The distortion of electron cloud of the anion by the cation is called polarisation). The Li+ ion is very small in size and has high tendency to distort electron cloud around the negative halide ion. Since anion with large size can be easily distorted, among halides, lithium iodide is the most covalent in nature.
(v) Reducing nature: The alkali metals are strong reducing agents, lithium being the most and sodium the least powerful
(Table 10.1). The standard electrode potential (E) which measures the reducing power represents the overall change :
With the small size of its ion, lithium has the highest hydration enthalpy which accounts for its high negative E value and its high reducing power.
Problem 10.2
The E for Cl2/Cl– is +1.36, for I2/I– is
+ 0.53, for Ag+ /Ag is +0.79, Na+ /Na is
–2.71 and for Li+ /Li is – 3.04. Arrange the following ionic species in decreasing order of reducing strength:
I–, Ag, Cl–, Li, Na
Solution
The order is Li > Na > I– > Ag > Cl–
(vi) Solutions in liquid ammonia: The alkali metals dissolve in liquid ammonia giving deep blue solutions which are conducting in nature.
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution. The solutions are paramagnetic and on standing slowly liberate hydrogen resulting in the formation of amide.
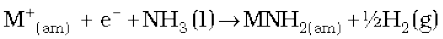
(where ‘am’ denotes solution in ammonia.)
In concentrated solution, the blue colour changes to bronze colour and becomes diamagnetic.