The simplest boron hydride known, is diborane. It is prepared by treating boron trifluoride with LiAlH4 in diethyl ether.
4BF3 + 3 LiAlH4 → 2B2H6 + 3LiF + 3AlF3
A convenient laboratory method for the preparation of diborane involves the oxidation of sodium borohydride with iodine.
2NaBH4 + I2 → B2H6 + 2NaI + H2
Diborane is produced on an industrial scale by the reaction of BF3 with sodium hydride.
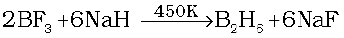
Diborane is a colourless, highly toxic gas with a b.p. of 180 K. Diborane catches fire spontaneously upon exposure to air. It burns in oxygen releasing an enormous amount of energy.
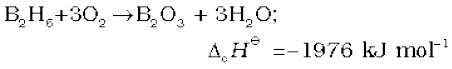
Most of the higher boranes are also spontaneously flammable in air. Boranes are readily hydrolysed by water to give boric acid.
B2H6(g) + 6H2O(l) → 2B(OH)3(aq) + 6H2(g)
Diborane undergoes cleavage reactions with Lewis bases(L) to give borane adducts, BH3⋅L
B2H6 + 2 NMe3 → 2BH3⋅NMe3
B2H6 + 2 CO → 2BH3⋅CO
Reaction of ammonia with diborane gives initially B2H6.2NH3 which is formulated as [BH2(NH3)2]+ [BH4]– ; further heating gives borazine, B3N3H6 known as “inorganic benzene” in view of its ring structure with alternate BH and NH groups.

The structure of diborane is shown in Fig.11.2(a). The four terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane, there are two bridging hydrogen atoms. The four terminal B-H bonds are regular two centre-two electron bonds while the two bridge (B-H-B) bonds are different and can be described in terms of three centre–two electron bonds shown in Fig.11.2 (b).
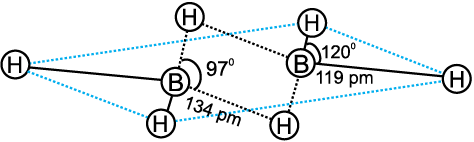
Fig.11.2(a) The structure of diborane, B2H6
Boron also forms a series of hydridoborates; the most important one is the tetrahedral [BH4]– ion. Tetrahydridoborates of several metals are known. Lithium and sodium tetra-hydridoborates, also known as borohydrides, are prepared by the reaction of metal hydrides with B2H6 in diethyl ether.
2MH + B2H6 → 2 M+ [BH4]– (M = Li or Na)
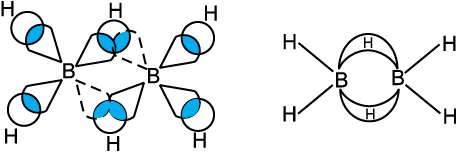
Fig.11.2(b) Bonding in diborane. Each B atom uses sp3 hybrids for bonding. Out
of the four sp3 hybrids on each B atom, one is without an electron shown in broken lines. The terminal B-H bonds are normal 2-centre-2-electron bonds but the two bridge bonds are 3-centre-2-electron bonds. The 3-centre-2-electron bridge bonds are also referred to as banana bonds.
Both LiBH4 and NaBH4 are used as reducing agents in organic synthesis. They are useful starting materials for preparing other metal borohydrides.