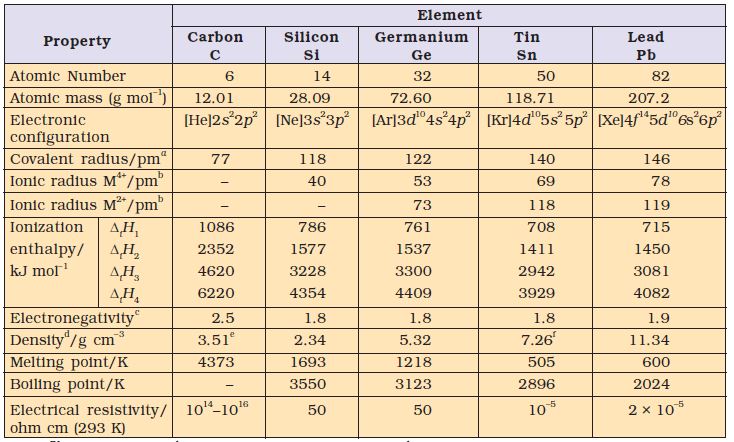
There is a considerable increase in covalent radius from C to Si, thereafter from Si to Pb a small increase in radius is observed. This is due to the presence of completely filled d and f orbitals in heavier members.
Table 11.3 Atomic and Physical Properties of Group 14 Elements
afor MIV oxidation state; b 6–coordination; c Pauling scale; d 293 K; e for diamond; for graphite, density is 2.22; fβ-form (stable at room temperature)
The first ionization enthalpy of group 14 members is higher than the corresponding members of group 13. The influence of inner core electrons is visible here also. In general the ionisation enthalpy decreases down the group. Small decrease in ∆iH from Si to Ge to Sn and slight increase in ∆iH from Sn to Pb is the consequence of poor shielding effect of intervening d and f orbitals and increase in size of the atom.
Due to small size, the elements of this group are slightly more electronegative than group 13 elements. The electronegativity values for elements from Si to Pb are almost the same.
All members of group14 are solids. Carbon and silicon are non-metals, germanium is a metalloid, whereas tin and lead are soft metals with low melting points. Melting points and boiling points of group 14 elements are much higher than those of corresponding elements of group 13.

© 2026 GoodEd Technologies Pvt. Ltd.