Carbon exhibits many allotropic forms; both crystalline as well as amorphous. Diamond and graphite are two well-known crystalline forms of carbon. In 1985, third form of carbon known as fullerenes was discovered by H.W.Kroto, E.Smalley and R.F.Curl. For this discovery they were awarded the Nobel Prize in 1996.
It has a crystalline lattice. In diamond each carbon atom undergoes sp3 hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion. The C–C bond length is 154 pm. The structure extends in space and produces a rigid three-dimensional network of carbon atoms. In this structure (Fig. 11.3) directional covalent bonds are present throughout the lattice.
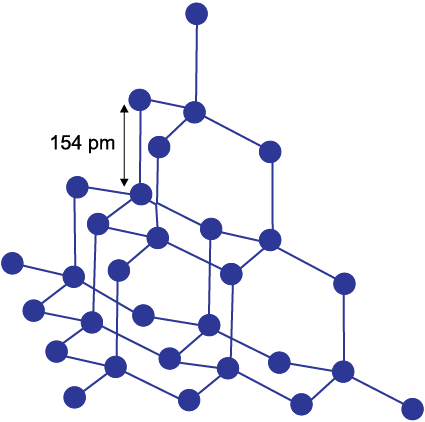
Fig. 11.3 The structure of diamond
It is very difficult to break extended covalent bonding and, therefore, diamond is a hardest substance on the earth. It is used as an abrasive for sharpening hard tools, in making dyes and in the manufacture of tungsten filaments for electric light bulbs.
Problem 11.7
Diamond is covalent, yet it has high melting point. Why ?
Solution
Diamond has a three-dimensional network involving strong C—C bonds, which are very difficult to break and, in turn has high melting point.
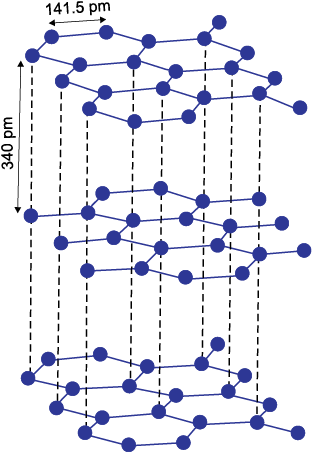
Fig 11.4 The structure of graphite

© 2026 GoodEd Technologies Pvt. Ltd.