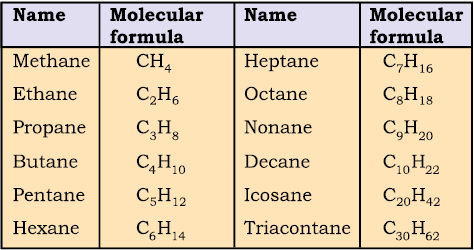
Straight chain hydrocarbons: The names of such compounds are based on their chain structure, and end with suffix ‘-ane’ and carry a prefix indicating the number of carbon atoms present in the chain (except from CH4 to C4H10, where the prefixes are derived from trivial names). The IUPAC names of some straight chain saturated hydrocarbons are given in Table 12.2. The alkanes in Table 12.2 differ from each other by merely the number of -CH2 groups in the chain. They are homologues of alkane series.
Table 12.2 IUPAC Names of Some Unbranched Saturated Hydrocarbons
Branched chain hydrocarbons: In a branched chain compound small chains of carbon atoms are attached at one or more carbon atoms of the parent chain. The small carbon chains (branches) are called alkyl groups. For example:
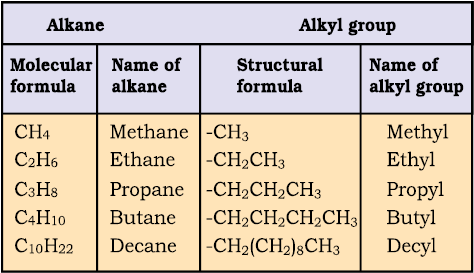
In order to name such compounds, the names of alkyl groups are prefixed to the name of parent alkane. An alkyl group is derived from a saturated hydrocarbon by removing a hydrogen atom from carbon. Thus, CH4 becomes -CH3 and is called methyl group. An alkyl group is named by substituting ‘yl’ for ‘ane’ in the corresponding alkane. Some alkyl groups are listed in Table 12.3.
Table 12.3 Some Alkyl Groups
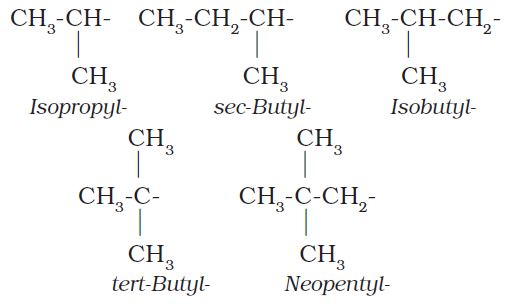
Common branched groups have specific trivial names. For example, the propyl groups can either be n-propyl group or isopropyl group. The branched butyl groups are called sec-butyl, isobutyl and tert-butyl group. We also encounter the structural unit, –CH2C(CH3)3, which is called neopentyl group.
Nomenclature of branched chain alkanes: We encounter a number of branched chain alkanes. The rules for naming them are given below.
1. First of all, the longest carbon chain in the molecule is identified. In the example (I) given below, the longest chain has nine carbons and it is considered as the parent or root chain. Selection of parent chain as shown in (II) is not correct because it has only eight carbons.
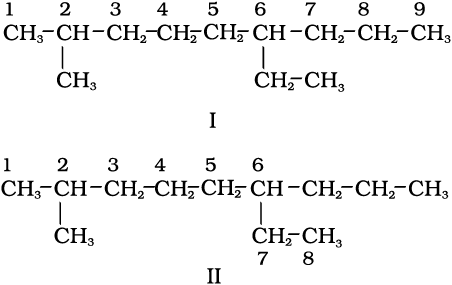
2. The carbon atoms of the parent chain are numbered to identify the parent alkane and to locate the positions of the carbon atoms at which branching takes place due to the substitution of alkyl group in place of hydrogen atoms. The numbering is done in such a way that the branched carbon atoms get the lowest possible numbers. Thus, the numbering in the above example should be from left to right (branching at carbon atoms 2 and 6) and not from right to left (giving numbers 4 and 8 to the carbon atoms at which branches are attached).
3. The names of alkyl groups attached as a branch are then prefixed to the name of the parent alkane and position of the substituents is indicated by the appropriate numbers. If different alkyl groups are present, they are listed in alphabetical order. Thus, name for the compound shown above is: 6-ethyl-2-methylnonane. [Note: the numbers are separated from the groups by hyphens and there is no break between methyl and nonane.]
4. If two or more identical substituent groups are present then the numbers are separated by commas. The names of identical substituents are not repeated, instead prefixes such as di (for 2), tri (for 3), tetra (for 4), penta (for 5), hexa (for 6) etc. are used. While writing the name of the substituents in alphabetical order, these prefixes, however, are not considered. Thus, the following compounds are named as:
5. If the two substituents are found in equivalent positions, the lower number is given to the one coming first in the alphabetical listing. Thus, the following compound is 3-ethyl-6-methyloctane and not 6-ethyl-3-methyloctane.
6. The branched alkyl groups can be named by following the above mentioned procedures. However, the carbon atom of the branch that attaches to the root alkane is numbered 1 as exemplified below.
The name of such branched chain alkyl group is placed in parenthesis while naming the compound. While writing the trivial names of substituents’ in alphabetical order, the prefixes iso- and neo- are considered to be the part of the fundamental name of alkyl group. The prefixes sec- and tert- are not considered to be the part of the fundamental name. The use of iso and related common prefixes for naming alkyl groups is also allowed by the IUPAC nomenclature as long as these are not further substituted. In multi-substituted compounds, the following rules may aso be remembered:
• If there happens to be two chains of equal size, then that chain is to be selected which contains more number of side chains.
• After selection of the chain, numbering is to be done from the end closer to the substituent.
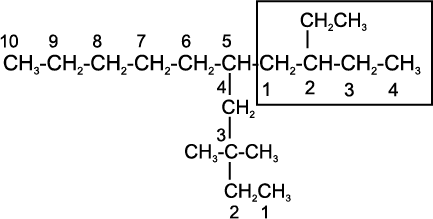
5-(2-Ethylbutyl)-3,3-dimethyldecane
[and not 5-(2,2-Dimethylbutyl)-3-ethyldecane]
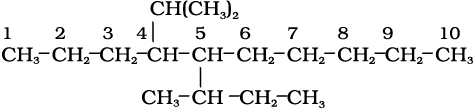
5-sec-Butyl-4-isopropyldecane
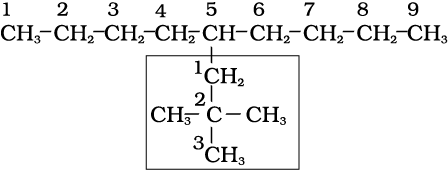
5-(2,2-Dimethylpropyl)nonane
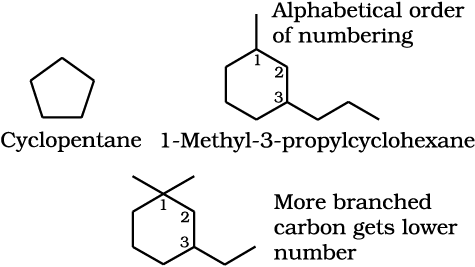
3-Ethyl-1,1-dimethylcyclohexane
(not 1-ethyl-3,3-dimethylcyclohexane)
Problem 12.7
Structures and IUPAC names of some hydrocarbons are given below. Explain why the names given in the parentheses are incorrect.
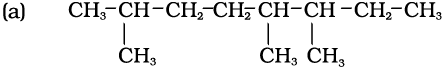
2,5,6- Trimethyloctane
[and not 3,4,7-Trimethyloctane]
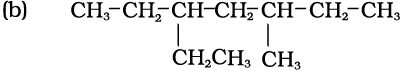
3-Ethyl-5-methylheptane
[and not 5-Ethyl-3-methylheptane]
Solution
(a) Lowest locant number, 2,5,6 is lower than 3,5,7, (b) substituents are in equivalent position; lower number is given to the one that comes first in the name according to alphabetical order.