In an organic reaction, the organic molecule (also referred as a substrate) reacts with an appropriate attacking reagent and leads to the formation of one or more intermediate(s) and finally product(s)
The general reaction is depicted as follows :
Substrate is that reactant which supplies carbon to the new bond and the other reactant is called reagent. If both the reactants supply carbon to the new bond then choice is arbitrary and in that case the molecule on which attention is focused is called substrate.
In such a reaction a covalent bond between two carbon atoms or a carbon and some other atom is broken and a new bond is formed. A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as reaction mechanism. The knowledge of reaction mechanism helps in understanding the reactivity of organic compounds and in planning strategy for their synthesis.
In the following sections, we shall learn some of the principles that explain how these reactions take place.
A covalent bond can get cleaved either by : (i) heterolytic cleavage, or by (ii) homolytic cleavage.
In heterolytic cleavage, the bond breaks in such a fashion that the shared pair of electrons remains with one of the fragments.
After heterolysis, one atom has a sextet electronic structure and a positive charge and the other, a valence octet with at least one lone pair and a negative charge. Thus, heterolytic cleavage of bromomethane will give 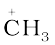 and Br– as shown below.
and Br– as shown below.
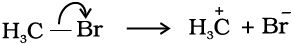
A species having a carbon atom possessing sextext of electrons and a positive charge is called a carbocation (earlier called carbonium ion). The 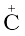 H3 ion is known as a methyl cation or methyl carbonium ion. Carbocations are classified as primary, secondary or tertiary depending on whether one, two or three carbons are directly attached to the positively charged carbon. Some other examples of carbocations are: CH3C+H2 (ethyl cation, a primary carbocation), (CH3)2C+H (isopropyl cation, a secondary carbocation), and (CH3)3C+ (tert-butyl cation, a tertiary carbocation). Carbocations are highly unstable and reactive species. Alkyl groups directly attached to the positively charged carbon stabilise the carbocations due to inductive and hyperconjugation effects, which you will be studying in the sections 12.7.5 and 12.7.9. The observed order of carbocation stability is: C+H3 < CH3C+H2 < (CH3)2C+H < (CH3)3C+. These carbocations have trigonal planar shape with positively charged carbon being sp2 hybridised. Thus, the shape of C+H3 may be considered as being derived from the overlap of three equivalent C(sp2) hybridised orbitals with 1s orbital of each of the three hydrogen atoms. Each bond may be represented as C(sp2)–H(1s) sigma bond. The remaining carbon orbital is perpendicular to the molecular plane and contains no electrons. [Fig. 12.3(a)].
H3 ion is known as a methyl cation or methyl carbonium ion. Carbocations are classified as primary, secondary or tertiary depending on whether one, two or three carbons are directly attached to the positively charged carbon. Some other examples of carbocations are: CH3C+H2 (ethyl cation, a primary carbocation), (CH3)2C+H (isopropyl cation, a secondary carbocation), and (CH3)3C+ (tert-butyl cation, a tertiary carbocation). Carbocations are highly unstable and reactive species. Alkyl groups directly attached to the positively charged carbon stabilise the carbocations due to inductive and hyperconjugation effects, which you will be studying in the sections 12.7.5 and 12.7.9. The observed order of carbocation stability is: C+H3 < CH3C+H2 < (CH3)2C+H < (CH3)3C+. These carbocations have trigonal planar shape with positively charged carbon being sp2 hybridised. Thus, the shape of C+H3 may be considered as being derived from the overlap of three equivalent C(sp2) hybridised orbitals with 1s orbital of each of the three hydrogen atoms. Each bond may be represented as C(sp2)–H(1s) sigma bond. The remaining carbon orbital is perpendicular to the molecular plane and contains no electrons. [Fig. 12.3(a)].
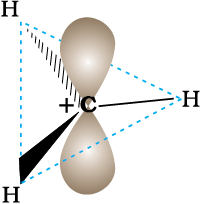
Fig. 12.3 (a) Shape of methyl carbocation
The heterolytic cleavage can also give a species in which carbon gets the shared pair of electrons. For example, when group Z attached to the carbon leaves without

electron pair, the methyl anion 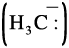 is
is
formed. Such a carbon species carrying a negative charge on carbon atom is called carbanion. Carbon in carbanion is generally sp3 hybridised and its structure is distorted tetrahedron as shown in Fig. 12.3(b).

Fig. 12.3 (b) Shape of methyl carbocation
Carbanions are also unstable and reactive species. The organic reactions which proceed through heterolytic bond cleavage are called ionic or heteropolar or just polar reactions.
In homolytic cleavage, one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms. Thus, in homolytic cleavage, the movement of a single electron takes place instead of an electron pair. The single electron movement is shown by ‘half-headed’ (fish hook: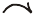 ) curved arrow. Such cleavage results in the formation of neutral species (atom or group) which contains an unpaired electron. These species are called free radicals. Like carbocations and carbanions, free radicals are also very reactive. A homolytic cleavage can be shown as:
) curved arrow. Such cleavage results in the formation of neutral species (atom or group) which contains an unpaired electron. These species are called free radicals. Like carbocations and carbanions, free radicals are also very reactive. A homolytic cleavage can be shown as:
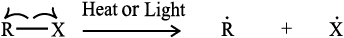
Alkyl
free radical
Alkyl radicals are classified as primary, secondary, or tertiary. Alkyl radical stability increases as we proceed from primary to tertiary:
Organic reactions, which proceed by homolytic fission are called free radical or homopolar or nonpolar reactions.