Hyperconjugation is a general stabilising interaction. It involves delocalisation of σ electrons of C—H bond of an alkyl group directly attached to an atom of unsaturated system or to an atom with an unshared p orbital. The σ electrons of C—H bond of the alkyl group enter into partial conjugation with the attached unsaturated system or with the unshared p orbital. Hyperconjugation is a permanent effect.
To understand hyperconjugation effect, let us take an example of 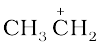 (ethyl cation) in which the positively charged carbon atom has an empty p orbital. One of the C-H bonds of the methyl group can align in the plane of this empty p orbital and the electrons constituting the C-H bond in plane with this p orbital can then be delocalised into the empty p orbital as depicted in Fig. 12.4 (a).
(ethyl cation) in which the positively charged carbon atom has an empty p orbital. One of the C-H bonds of the methyl group can align in the plane of this empty p orbital and the electrons constituting the C-H bond in plane with this p orbital can then be delocalised into the empty p orbital as depicted in Fig. 12.4 (a).
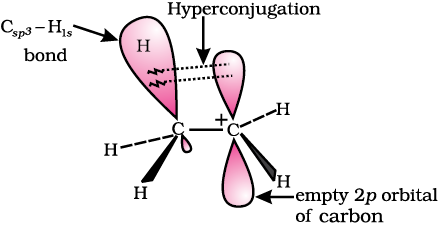
Fig. 12.4(a) Orbital diagram showing hyperconjugation in ethyl cation
In general, greater the number of alkyl groups attached to a positively charged carbon atom, the greater is the hyperconjugation interaction and stabilisation of the cation. Thus, we have the following relative stability of carbocations :
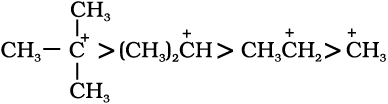
Hyperconjugation is also possible in alkenes and alkylarenes.
Delocalisation of electrons by hyperconjugation in the case of alkene can be depicted as in Fig. 12.4(b).
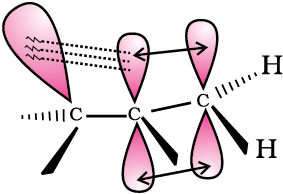
Fig. 12.4(b) Orbital diagram showing hyperconjugation in propene
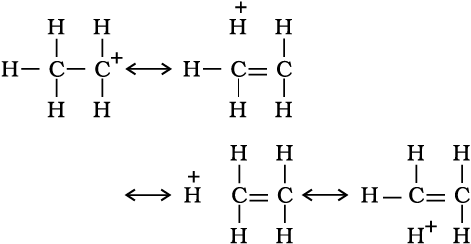
There are various ways of looking at the hyperconjugative effect. One of the way is to regard C—H bond as possessing partial ionic character due to resonance.
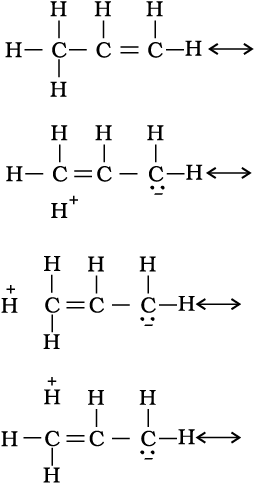
The hyperconjugation may also be regarded as no bond resonance.
Problem 12.19
Explain why (CH3)3C+ is more stable than is the least stable cation.
Solution
Hyperconjugation interaction in (CH3)3C+ is greater than in as the (CH3)3C+ has nine C-H bonds. In , vacant p orbital is perpendicular to the plane
in which C-H bonds lie; hence cannot overlap with it. Thus, lacks hyperconjugative stability.

© 2026 GoodEd Technologies Pvt. Ltd.