Chromatography is an important technique extensively used to separate mixtures into their components, purify compounds and also to test the purity of compounds. The name chromatography is based on the Greek word chroma, for colour since the method was first used for the separation of coloured substances found in plants. In this technique, the mixture of substances is applied onto a stationary phase, which may be a solid or a liquid. A pure solvent, a mixture of solvents, or a gas is allowed to move slowly over the stationary phase. The components of the mixture get gradually separated from one another. The moving phase is called the mobile phase.
Based on the principle involved, chromatography is classified into different categories. Two of these are:
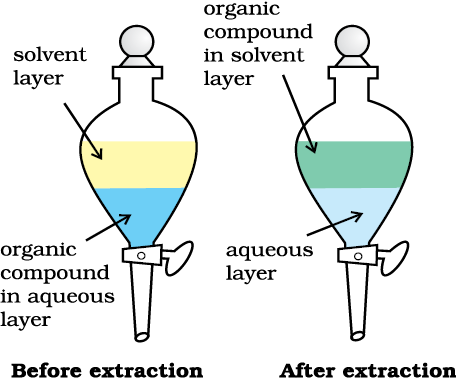
Fig.12.10 Differential extraction. Extraction of compound takes place based on difference in solubility
(a) Adsorption chromatography, and
(b) Partition chromatography.
a) Adsorption Chromatography: Adsor-ption chromatography is based on the fact that different compounds are adsorbed on an adsorbent to different degrees. Commonly used adsorbents are silica gel and alumina. When a mobile phase is allowed to move over a stationary phase (adsorbent), the components of the mixture move by varying distances over the stationary phase. Following are two main types of chromatographic techniques based on the principle of differential adsorption.
(a) Column chromatography, and
(b) Thin layer chromatography.
Column Chromatography: Column chromatography involves separation of a mixture over a column of adsorbent (stationary phase) packed in a glass tube. The column is fitted with a stopcock at its lower end (Fig. 12.11). The mixture adsorbed on adsorbent is placed on the top of the adsorbent column packed in a glass tube. An appropriate eluant which is a liquid or a mixture of liquids is allowed to flow down the column slowly. Depending upon the degree to which the compounds are adsorbed, complete separation takes place. The most readily adsorbed substances are retained near the top and others come down to various distances in the column (Fig.12.11).
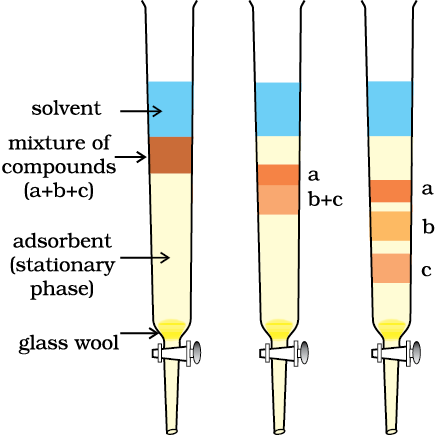
Fig.12.11 Column chromatography. Different stages of separation of components of a mixture.
Thin Layer Chromatography: Thin layer chromatography (TLC) is another type of adsorption chromatography, which involves separation of substances of a mixture over a thin layer of an adsorbent coated on glass plate. A thin layer (about 0.2mm thick) of an adsorbent (silica gel or alumina) is spread over a glass plate of suitable size. The plate is known as thin layer chromatography plate or chromaplate. The solution of the mixture to be separated is applied as a small spot about 2 cm above one end of the TLC plate. The glass plate is then placed in a closed jar containing the eluant (Fig. 12.12a). As the eluant rises up the plate, the components of the mixture move up along with the eluant to different distances depending on their degree of adsorption and separation takes place. The relative adsorption of each component of the mixture is expressed in terms of its retardation factor i.e. Rf value (Fig.12.12 b).
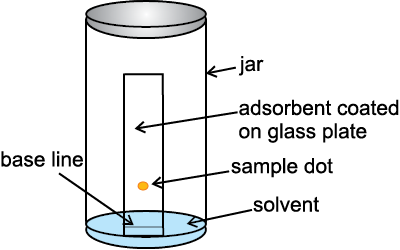
Fig.12.12 (a) Thin layer chromatography. Chromatogram being developed.
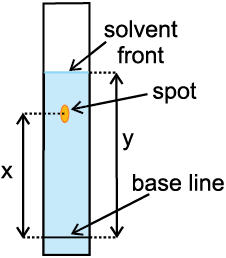
Fig.12.12 (b) Developed chromatogram.
Partition Chromatography: Partition chromatography is based on continuous differential partitioning of components of a mixture between stationary and mobile phases. Paper chromatography is a type
of partition chromatography. In paper chromatography, a special quality paper known as chromatography paper is used. Chromatography paper contains water trapped in it, which acts as the stationary phase.
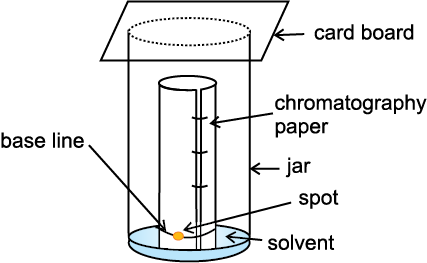
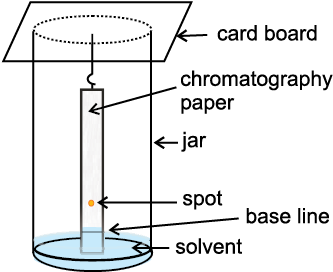
Fig.12.13 Paper chromatography. Chromatography paper in two different shapes.