Carius method: A known mass of an organic compound is heated with fuming nitric acid in the presence of silver nitrate contained in a hard glass tube known as Carius tube, (Fig.12.17)
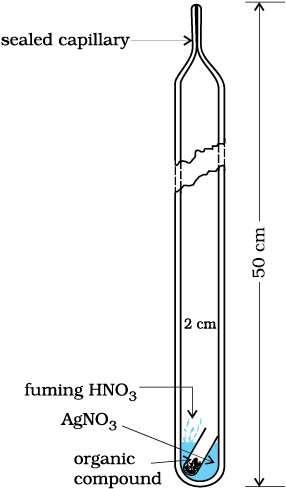
Fig.12.17 Carius method. Halogen containing organic compound is heated with fuming nitric acid in the presence of silver nitrate.
in a furnace. Carbon and hydrogen present in the compound are oxidised to carbon dioxide and water. The halogen present forms the corresponding silver halide (AgX). It is filtered, washed, dried and weighed.
Let the mass of organic
compound taken = m g
Mass of AgX formed = m1 g
1 mol of AgX contains 1 mol of X
Mass of halogen in m1g of AgX
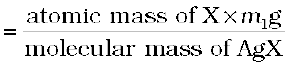
Percentage of halogen
Problem 12.23
In Carius method of estimation of halogen, 0.15 g of an organic compound gave 0.12 g of AgBr. Find out the percentage of bromine in the compound.
Solution
Molar mass of AgBr = 108 + 80
= 188 g mol-1
188 g AgBr contains 80 g bromine
0.12 g AgBr contains 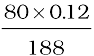 g bromine
g bromine

A known mass of an organic compound is heated in a Carius tube with sodium peroxide or fuming nitric acid. Sulphur present in the compound is oxidised to sulphuric acid. It is precipitated as barium sulphate by adding excess of barium chloride solution in water. The precipitate is filtered, washed, dried and weighed. The percentage of sulphur can be calculated from the mass of barium sulphate.
Let the mass of organic
compound taken = m g
and the mass of barium
sulphate formed = m1g
1 mol of BaSO4 = 233 g BaSO4 = 32 g sulphur
m1 g BaSO4 contains 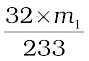 g sulphur
g sulphur

Problem 12.24
In sulphur estimation, 0.157 g of an organic compound gave 0.4813 g of barium sulphate. What is the percentage of sulphur in the compound?
Solution
Molecular mass of BaSO4 = 137+32+64
= 233 g
233 g BaSO4 contains 32 g sulphur
0.4813 g BaSO4 contains 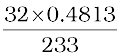 g sulphur
g sulphur
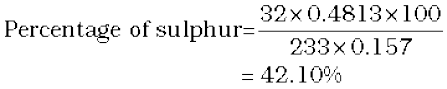
A known mass of an organic compound is heated with fuming nitric acid whereupon phosphorus present in the compound is oxidised to phosphoric acid. It is precipitated as ammonium phosphomolybdate, (NH4)3 PO4.12MoO3, by adding ammonia and ammonium molybdate. Alternatively, phosphoric acid may be precipitated as MgNH4PO4 by adding magnesia mixture which on ignition yields Mg2P2O7.
Let the mass of organic compound taken
= m g and mass of ammonium phospho molydate = m1g
Molar mass of (NH4)3PO4.12MoO3 = 1877g
Percentage of phosphorus =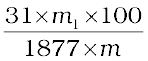 %
%
If phosphorus is estimated as Mg2P2O7,
Percentage of phosphorus =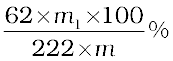
where, 222 u is the molar mass of Mg2P2O7, m, the mass of organic compound taken, m1, the mass of Mg2P2O7 formed and 62, the mass of two phosphorus atoms present in the compound Mg2P2O7.
The percentage of oxygen in an organic compound is usually found by difference between the total percentage composition (100) and the sum of the percentages of all other elements. However, oxygen can also be estimated directly as follows:
A definite mass of an organic compound is decomposed by heating in a stream of nitrogen gas. The mixture of gaseous products containing oxygen is passed over red-hot coke when all the oxygen is converted to carbon monoxide. This mixture is passed through warm iodine pentoxide (I2O5) when carbon monoxide is oxidised to carbon dioxide producing iodine.
Compound  O2 + other gaseous products
O2 + other gaseous products
2C + O2 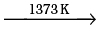 2CO]× 5 (A)
2CO]× 5 (A)
I2O5 + 5CO → I2 + 5CO2]× 2 (B)
On making the amount of CO produced in equation (A) equal to the amount of CO used in equation (B) by multiplying the equations (A) and (B) by 5 and 2 respectively; we find that each mole of oxygen liberated from the compound will produce two moles of carbondioxide.
Thus 88 g carbon dioxide is obtained if 32 g oxygen is liberated.
Let the mass of organic compound taken be m g
Mass of carbon dioxide produced be m1 g
∴ m1 g carbon dioxide is obtained from 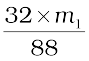 g O2
g O2
∴Percentage of oxygen = 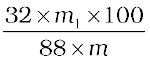 %
%
The percentage of oxygen can be derived from the amount of iodine produced also.
Presently, the estimation of elements in an organic compound is carried out by using microquantities of substances and automatic experimental techniques. The elements, carbon, hydrogen and nitrogen present in a compound are determined by an apparatus known as CHN elemental analyser. The analyser requires only a very small amount of the substance (1-3 mg) and displays the values on a screen within a short time. A detailed discussion of such methods is beyond the scope of this book.
Summary
In this unit, we have learnt some basic concepts in structure and reactivity of organic compounds, which are formed due to covalent bonding. The nature of the covalent bonding in organic compounds can be described in terms of orbitals hybridisation concept, according to which carbon can have sp3, sp2 and sp hybridised orbitals. The sp3, sp2 and sp hybridised carbons are found in compounds like methane, ethene and ethyne respectively. The tetrahedral shape of methane, planar shape of ethene and linear shape of ethyne can be understood on the basis of this concept. A sp3 hybrid orbital can overlap with 1s orbital of hydrogen to give a carbon - hydrogen (C–H) single bond (sigma, σ bond). Overlap of a sp2 orbital of one carbon with sp2 orbital of another results in the formation of a carbon–carbon σ bond. The unhybridised p orbitals on two adjacent carbons can undergo lateral (side-by-side) overlap to give a pi (π) bond. Organic compounds can be represented by various structural formulas. The three dimensional representation of organic compounds on paper can be drawn by wedge and dash formula.
Organic compounds can be classified on the basis of their structure or the functional groups they contain. A functional group is an atom or group of atoms bonded together in a unique fashion and which determines the physical and chemical properties of the compounds. The naming of the organic compounds is carried out by following a set of rules laid down by the International Union of Pure and Applied Chemistry (IUPAC). In IUPAC nomenclature, the names are correlated with the structure in such a way that the reader can deduce the structure from the name.
Organic reaction mechanism concepts are based on the structure of the substrate molecule, fission of a covalent bond, the attacking reagents, the electron displacement effects and the conditions of the reaction. These organic reactions involve breaking and making of covalent bonds. A covalent bond may be cleaved in heterolytic or homolytic fashion. A heterolytic cleavage yields carbocations or carbanions, while a homolytic cleavage gives free radicals as reactive intermediate. Reactions proceeding through heterolytic cleavage involve the complimentary pairs of reactive species. These are electron pair donor known as nucleophile and an electron pair acceptor known as electrophile. The inductive, resonance, electromeric and hyperconjugation effects may help in the polarisation of a bond making certain carbon atom or other atom positions as places of low or high electron densities.
Organic reactions can be broadly classified into following types; substitution, addition, elimination and rearrangement reactions.
Purification, qualitative and quantitative analysis of organic compounds are carried out for determining their structures. The methods of purification namely : sublimation, distillation and differential extraction are based on the difference in one or more physical properties. Chromatography is a useful technique of separation, identification and purification of compounds. It is classified into two categories : adsorption and partition chromatography. Adsorption chromatography is based on differential adsorption of various components of a mixture on an adsorbent. Partition chromatography involves continuous partitioning of the components of a mixture between stationary and mobile phases. After getting the compound in a pure form, its qualitative analysis is carried out for detection of elements present in it. Nitrogen, sulphur, halogens and phosphorus are detected by Lassaigne’s test. Carbon and hydrogen are estimated by determining the amounts of carbon dioxide and water produced. Nitrogen is estimated by Dumas or Kjeldahl’s method and halogens by Carius method. Sulphur and phosphorus are estimated by oxidising them to sulphuric and phosphoric acids respectively. The percentage of oxygen is usually determined by difference between the total percentage (100) and the sum of percentages of all other elements present.