Physical properties of alkynes follow the same trend of alkenes and alkanes. First three members are gases, the next eight are liquids and the higher ones are solids. All alkynes are colourless. Ethyene has characteristic odour. Other members are odourless. Alkynes are weakly polar in nature. They are lighter than water and immiscible with water but soluble in organic solvents like ethers, carbon tetrachloride and benzene. Their melting point, boiling point and density increase with increase in molar mass.
Alkynes show acidic nature, addition reactions and polymerisation reactions as follows :
A. Acidic character of alkyne: Sodium metal and sodamide (NaNH2) are strong bases. They react with ethyne to form sodium acetylide with the liberation of dihydrogen gas. These reactions have not been observed in case of ethene and ethane thus indicating that ethyne is acidic in nature in comparison to ethene and ethane. Why is it so ? Has it something to do with their structures and the hybridisation ? You have read that hydrogen atoms in ethyne are attached to the sp hybridised carbon atoms whereas they are attached to sp2 hybridised carbon atoms in ethene and sp3 hybridised carbons in ethane. Due to the maximum percentage of s character (50%), the sp hybridised orbitals of carbon atoms in ethyne molecules have highest electronegativity; hence, these attract the shared electron pair of the C-H bond of ethyne to a greater extent than that of the sp2 hybridised orbitals of carbon in ethene and the sp3 hybridised orbital of carbon in ethane. Thus in ethyne, hydrogen atoms can be liberated as protons more easily as compared to ethene and ethane. Hence, hydrogen atoms of ethyne attached to triply bonded carbon atom are acidic in nature. You may note that the hydrogen atoms attached to the triply bonded carbons are acidic but not all the hydrogen atoms of alkynes.
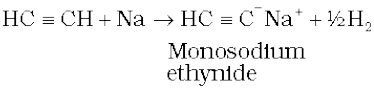 (13.59)
(13.59)
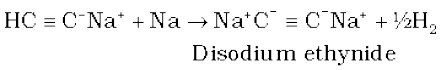 (13.60)
(13.60)
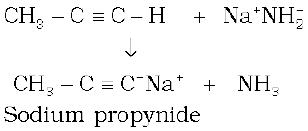 (13.61)
(13.61)
These reactions are not shown by alkenes and alkanes, hence used for distinction between alkynes, alkenes and alkanes. What about the above reactions with but-1-yne and but-2-yne ? Alkanes, alkenes and alkynes follow the following trend in their acidic behaviour :
i) 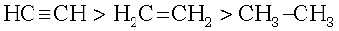
ii) 
B. Addition reactions: Alkynes contain a triple bond, so they add up, two molecules of dihydrogen, halogen, hydrogen halides etc. Formation of the addition product takes place according to the following steps.
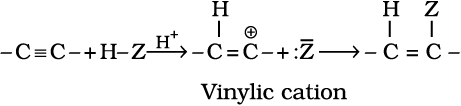
The addition product formed depends upon stability of vinylic cation. Addition in unsymmetrical alkynes takes place according to Markovnikov rule. Majority of the reactions of alkynes are the examples of electrophilic addition reactions. A few addition reactions are given below:
(i) Addition of dihydrogen
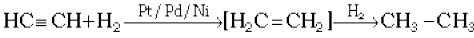 (13.62)
(13.62)
 (13.63)
(13.63)
(ii) Addition of halogens
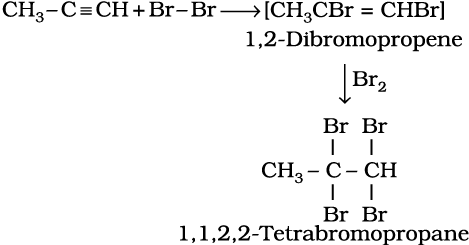 (13.64)
(13.64)
Reddish orange colour of the solution of bromine in carbon tetrachloride is decolourised. This is used as a test for unsaturation.
(iii) Addition of hydrogen halides
Two molecules of hydrogen halides (HCl, HBr, HI) add to alkynes to form gem dihalides (in which two halogens are attached to the same carbon atom)
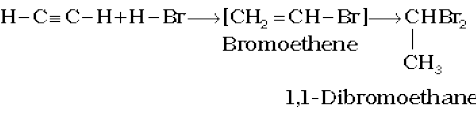 (13.65)
(13.65)
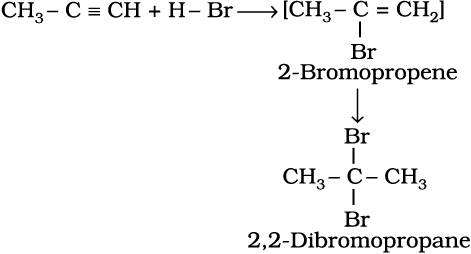 (13.66)
(13.66)
(iv) Addition of water
Like alkanes and alkenes, alkynes are also immiscible and do not react with water. However, one molecule of water adds to alkynes on warming with mercuric sulphate and dilute sulphuric acid at 333 K to form carbonyl compounds.
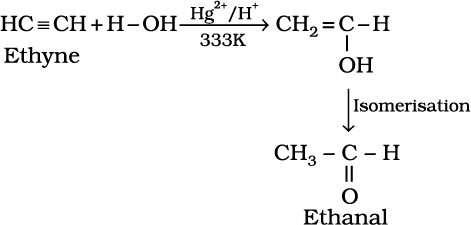 (13.67)
(13.67)
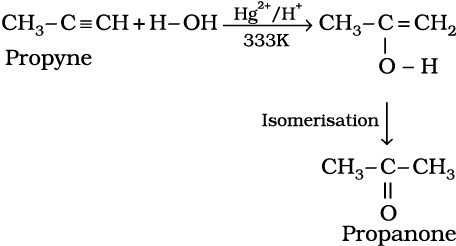 (13.68)
(13.68)
(v) Polymerisation
(a) Linear polymerisation: Under suitable conditions, linear polymerisation of ethyne takes place to produce polyacetylene or polyethyne which is a high molecular weight polyene containing repeating units of
(CH = CH – CH = CH ) and can be represented as —(CH = CH – CH = CH)n— Under special conditions, this polymer conducts electricity. Thin film of polyacetylene can be used as electrodes in batteries. These films are good conductors, lighter and cheaper than the metal conductors.
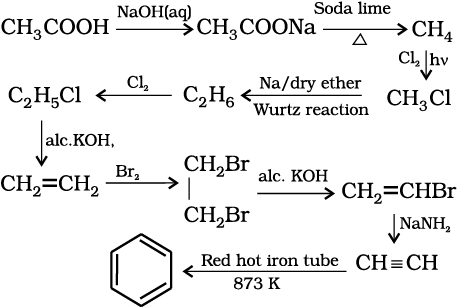
(b) Cyclic polymerisation: Ethyne on passing through red hot iron tube at 873K undergoes cyclic polymerization. Three molecules polymerise to form benzene, which is the starting molecule for the preparation of derivatives of benzene, dyes, drugs and large number of other organic compounds. This is the best route for entering from aliphatic to aromatic compounds as discussed below:
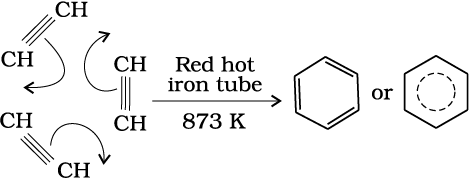 (13.69)
(13.69)
Problem 13.14
How will you convert ethanoic acid into benzene?
Solution