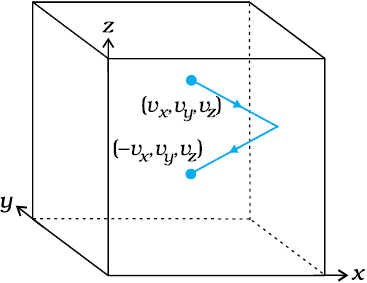
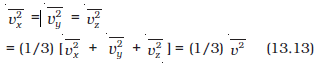To calculate the force (and pressure) on the wall, we need to calculate momentum imparted to the wall per unit time. In a small time interval , a molecule with x-component of velocity vx will hit the wall if it is within the distance vx from the wall. That is, all molecules within the volume Avx only can hit the wall in time Dt. But, on the average, half of these are moving towards the wall and the other half away from the wall. Thus, the number of molecules with velocity (vx, vy, vz ) hitting the wall in time is
½A vx n, where n is the number of molecules per unit volume. The total momentum transferred to the wall by these molecules in time is:
Q = (2mvx) (½ n A vx ) (13.10)
The force on the wall is the rate of momentum transfer Q/ and pressure is force per unit area :
P = Q /(A ) = n m vx 2 (3.11)
Actually, all molecules in a gas do not have the same velocity; there is a distribution in velocities. The above equation, therefore, stands for pressure due to the group of molecules with speed vx in the x-direction and n stands for the number density of that group of molecules. The total pressure is obtained by summing over the contribution due to all groups:
P = n m vx2 (13.12)
where is the average of vx2 . Now the gas is isotropic, i.e. there is no preferred direction of velocity of the molecules in the vessel.
Therefore, by symmetry,
where v is the speed and v2 denotes the mean of the squared speed. Thus
P = (1/3) n m (13.14)
Some remarks on this derivation. First, though we choose the container to be a cube, the shape of the vessel really is immaterial. For a vessel of arbitrary shape, we can always choose a small infinitesimal (planar) area and carry through the steps above. Notice that both A and do not appear in the final result. By Pascal’s law, given in Ch. 10, pressure in one portion of the gas in equilibrium is the same as anywhere else. Second, we have ignored any collisions inthe derivation. Though this assumption is difficult to justify rigorously, we can qualitatively see that it will not lead to erroneous results.
The number of molecules hitting the wall in time Dt was found to be ½ n Avx. Now the collisions are random and the gas is in a steady state. Thus, if a molecule with velocity (vx, vy, vz ) acquires a different velocity due to collision with some molecule, there will always be some other molecule with a different initial velocity which after a collision acquires the velocity (vx, vy, vz ).
If this were not so, the distribution of velocities would not remain steady. In any case we are finding . Thus, on the whole, molecular collisions (if they are not too frequent and the time spent in a collision is negligible compared to time between collisions) will not affect the calculation above.