There are several methods of introducing the ligated DNA into recipient cells. Recipient cells after making them ‘competent’ to receive, take up DNA present in its surrounding. So, if a recombinant DNA bearing gene for resistance to an antibiotic (e.g., ampicillin) is transferred into E. coli cells, the host cells become transformed into ampicillin-resistant cells. If we spread the transformed cells on agar plates containing ampicillin, only transformants will grow, untransformed recipient cells will die. Since, due to ampicillin resistance gene, one is able to select a transformed cell in the presence of ampicillin. The ampicillin resistance gene in this case is called a selectable marker.
When you insert a piece of alien DNA into a cloning vector and transfer it into a bacterial, plant or animal cell, the alien DNA gets multiplied. In almost all recombinant technologies, the ultimate aim is to produce a desirable protein. Hence, there is a need for the recombinant DNA to be expressed. The foreign gene gets expressed under appropriate conditions. The expression of foreign genes in host cells involve understanding many technical details.
After having cloned the gene of interest and having optimised the conditions to induce the expression of the target protein, one has to consider producing it on a large scale. Can you think of any reason why there is a need for large-scale production? If any protein encoding gene is expressed in a heterologous host, it is called a recombinant protein. The cells harbouring cloned genes of interest may be grown on a small scale in the laboratory. The cultures may be used for extracting the desired protein and then purifying it by using different separation techniques.
The cells can also be multiplied in a continuous culture system wherein the used medium is drained out from one side while fresh medium is added from the other to maintain the cells in their physiologically most active log/exponential phase. This type of culturing method produces a larger biomass leading to higher yields of desired protein.
Small volume cultures cannot yield appreciable quantities of products. To produce in large quantities, the development of bioreactors, where large volumes (100-1000 litres) of culture can be processed, was required. Thus, bioreactors can be thought of as vessels in which raw materials are biologically converted into specific products, individual enzymes, etc., using microbial plant, animal or human cells. A bioreactor provides the optimal conditions for achieving the desired product by providing optimum growth conditions (temperature, pH, substrate, salts, vitamins, oxygen).
The most commonly used bioreactors are of stirring type, which are shown in Figure 11.7.
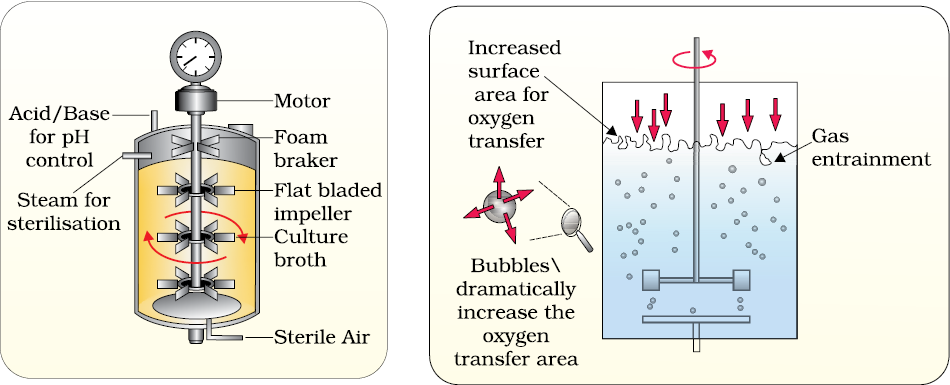
A stirred-tank reactor is usually cylindrical or with a curved base to facilitate the mixing of the reactor contents. The stirrer facilitates even mixing and oxygen availability throughout the bioreactor. Alternatively air can be bubbled through the reactor. If you look at the figure closely you will see that the bioreactor has an agitator system, an oxygen delivery system and a foam control system, a temperature control system, pH control system and sampling ports so that small volumes of the culture can be withdrawn periodically.
After completion of the biosynthetic stage, the product has to be subjected through a series of processes before it is ready for marketing as a finished product. The processes include separation and purification, which are collectively referred to as downstream processing. The product has to be formulated with suitable preservatives. Such formulation has to undergo thorough clinical trials as in case of drugs. Strict quality control testing for each product is also required. The downstream processing and quality control testing vary from product to product.