This theory explains the mechanism of heterogeneous catalysis. The old theory, known as adsorption theory of catalysis, was that the reactants in gaseous state or in solutions, are adsorbed on the surface of the solid catalyst. The increase in concentration of the reactants on the surface increases the rate of reaction. Adsorption being an exothermic process, the heat of adsorption is utilised in enhancing the rate of the reaction.
The catalytic action can be explained in terms of the intermediate compound formation, the theory of which you have already studied in Section 4.5.1
The modern adsorption theory is the combination of intermediate compound formation theory and the old adsorption theory. The catalytic activity is localised on the surface of the catalyst. The mechanism involves five steps:
(i) Diffusion of reactants to the surface of the catalyst.
(ii) Adsorption of reactant molecules on the surface of the catalyst.
(iii) Occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate (Fig. 5.3).
(iv) Desorption of reaction products from the catalyst surface, and thereby, making the surface available again for more reaction to occur.
(v) Diffusion of reaction products away from the catalyst’s surface. The surface of the catalyst unlike the inner part of the bulk, has free valencies which provide the seat for chemical forces of attraction. When a gas comes in contact with such a surface, its molecules are held up there due to loose chemical combination. If different molecules are adsorbed side by side, they may react with each other resulting in the formation of new molecules. Thus, formed molecules may evaporate leaving the surface for the fresh reactant molecules.
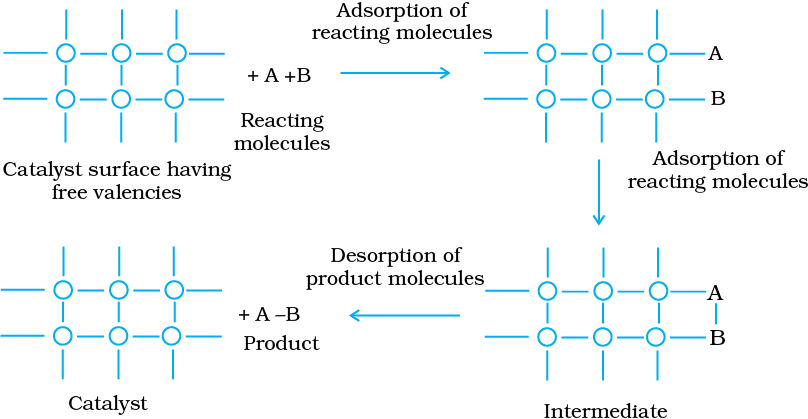
Fig. 5.3 Adsorption of reacting molecules, formation of intermediate and desorption of products
Important features of solid catalysts
(a) Activity
The activity of a catalyst depends upon the strength of chemisorption to a large extent. The reactants must get adsorbed reasonably strongly on to the catalyst to become active. However, they must not get adsorbed so strongly that they are immobilised and other reactants are left with no space on the catalyst’s surface for adsorption. It has been found that for hydrogenation reaction, the catalytic activity increases from Group 5 to Group 11 metals with maximum activity being shown by groups 7-9 elements of the periodic table (Class XI, Unit 3).
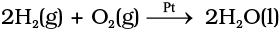
(b) Selectivity
The selectivity of a catalyst is its ability to direct a reaction to yield a particular product selectively, when under the same reaction conditions many products are possible. Selectivity of different catalysts for same reactants is different. For example, starting with H2 and CO, and using different catalysts, we get different products.
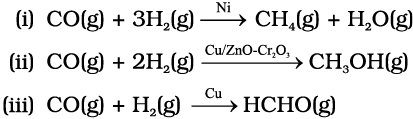
Thus, it can be inferred that the action of a catalyst is highly selective in nature. As a result a substance which acts as a catalyst in one reaction may fail to catalyse another reaction.