(a) Extraction of iron from its oxides
At 500 – 800 K (lower temperature range in the blast furnace),
3 Fe2O3 + CO → 2 Fe3O4 + CO2 (6.28)
Fe3O4 + 4 CO → 3Fe + 4 CO2 (6.29)
Fe2O3 + CO → 2FeO + CO2 (6.30)
At 900 – 1500 K (higher temperature range in the blast furnace):
C + CO2 → 2 CO (6.31)
FeO + CO → Fe + CO2 (6.32)
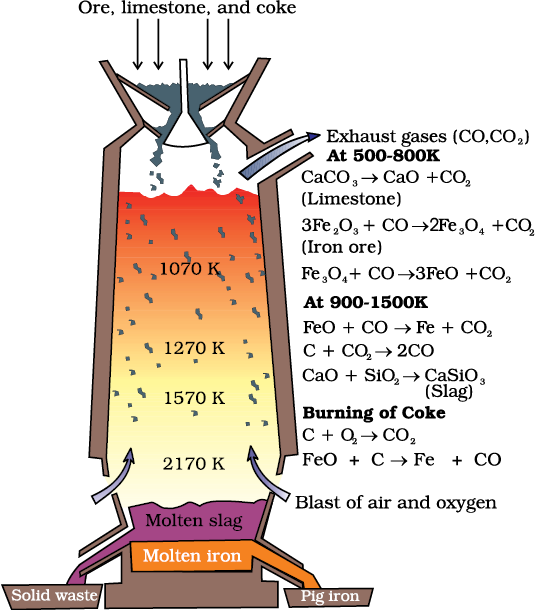
Fig. 6.5: Blast furnace
Limestone is also decomposed to CaO which removes silicate impurity of the ore as slag. The slag is in molten state and separates out from iron.
The iron obtained from Blast furnace contains about 4% carbon and many impurities in smaller amount (e.g., S, P, Si, Mn). This is known as pig iron and cast into variety of shapes. Cast iron is different from pig iron and is made by melting pig iron with scrap iron and coke using hot air blast. It has slightly lower carbon content (about 3%) and is extremely hard and brittle.
Further Reductions
Wrought iron or malleable iron is the purest form of commercial iron and is prepared from cast iron by oxidising impurities in a reverberatory furnace lined with haematite. The haematite oxidises carbon to carbon monoxide:
Fe2O3 + 3 C → 2 Fe + 3 CO (6.31)
Limestone is added as a flux and sulphur, silicon and phosphorus are oxidised and passed into the slag. The metal is removed and freed from the slag by passing through rollers.
(b) Extraction of copper from cuprous oxide [copper(I) oxide]
2Cu2S + 3O2 → 2Cu2O + 2SO2 (6.32)
The oxide can then be easily reduced to metallic copper using coke:
Cu2O + C → 2 Cu + CO (6.33)
In actual process, the ore is heated in a reverberatory furnace after mixing with silica. In the furnace, iron oxide ‘slags of’ as iron slicate is formed. Copper is produced in the form of copper matte. This contains Cu2S and FeS.
FeO + SiO2 → FeSiO3 (Slag) (6.34)
Copper matte is then charged into silica lined convertor. Some silica is also added and hot air blast is blown to convert the remaining FeS, FeO and Cu2S/Cu2O to the metallic copper. Following reactions take place:
2FeS + 3O2 → 2FeO + 2SO2 (6.35)
FeO + SiO2 → FeSiO3 (6.36)
2Cu2S + 3O2 → 2Cu2O + 2SO2 (6.37)
2Cu2O + Cu2S → 6Cu + SO2 (6.38)
The solidified copper obtained has blistered appearance due to the evolution of SO2 and so it is called blister copper.
(c) Extraction of zinc from zinc oxide
The reduction of zinc oxide is done using coke. The temperature in this case is higher than that in the case of copper. For the purpose of heating, the oxide is made into brickettes with coke and clay.
ZnO + C 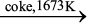 Zn + CO (6.39)
Zn + CO (6.39)
The metal is distilled off and collected by rapid chilling.
Intext Questions
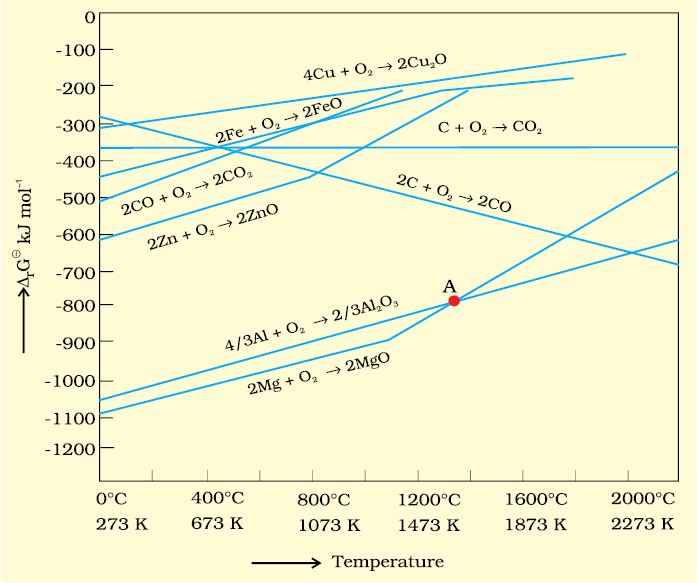
6.3 The reaction,
Cr2O3+2Al → Al2O3+2Cr (∆GƟ= – 421kJ)
6.4 Is it true that under certain conditions, Mg can reduce Al2O3 and Al can reduce MgO? What are those conditions?