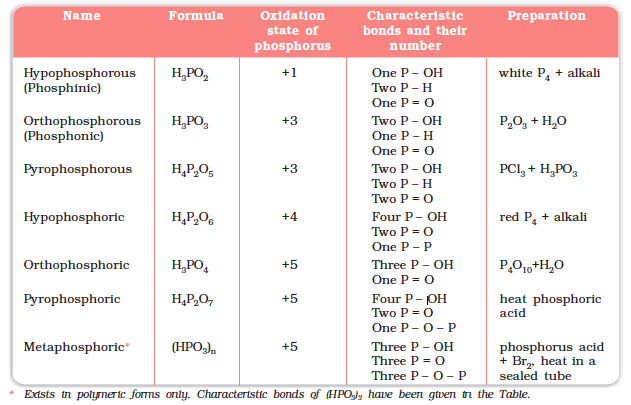
The compositions of the oxoacids are interrelated in terms of loss or gain of H2O molecule or O-atom. The structures of some important oxoacids are given next.
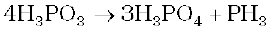
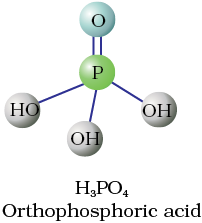
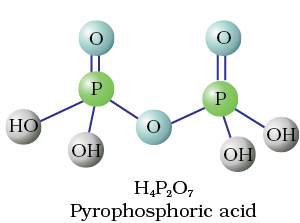
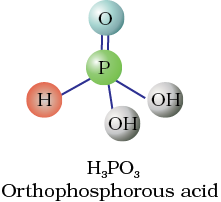
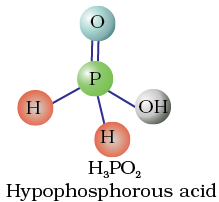
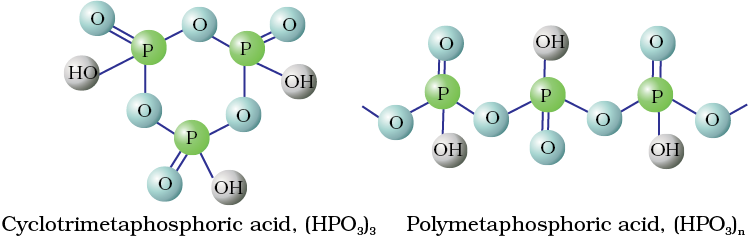
Fig. 7.4 Structures of some important oxoacids of phosphorus
The acids which contain P–H bond have strong reducing properties. Thus, hypophosphorous acid is a good reducing agent as it contains two P–H bonds and reduces, for example, AgNO3 to metallic silver.
4 AgNO3 + 2H2O + H3PO2 → 4Ag + 4HNO3 + H3PO4
These P–H bonds are not ionisable to give H+ and do not play any role in basicity. Only those H atoms which are attached with oxygen in P–OH form are ionisable and cause the basicity. Thus, H3PO3 and H3PO4 are dibasic and tribasic, respectively as the structure of H3PO3 has two P–OH bonds and H3PO4 three.
Example 7.9
How do you account for the reducing behaviour of H3PO2 on the basis of its structure?
Solution
In H3PO2, two H atoms are bonded directly to P atom which imparts reducing character to the acid.
Intext Questions
7.11 What is the basicity of H3PO4?
7.12 What happens when H3PO3 is heated?

© 2026 GoodEd Technologies Pvt. Ltd.