Nearly all the transition elements display typical metallic properties such as high tensile strength, ductility, malleability, high thermal and electrical conductivity and metallic lustre. With the exceptions of Zn, Cd, Hg and Mn, they have one or more typical metallic structures at normal temperatures.
Lattice Structures of Transition Metals
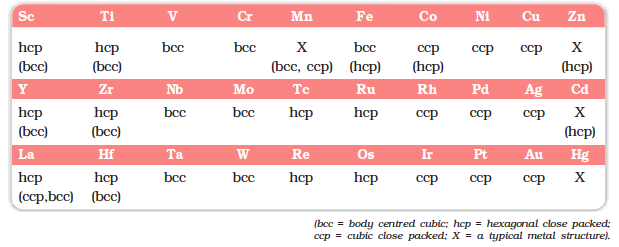
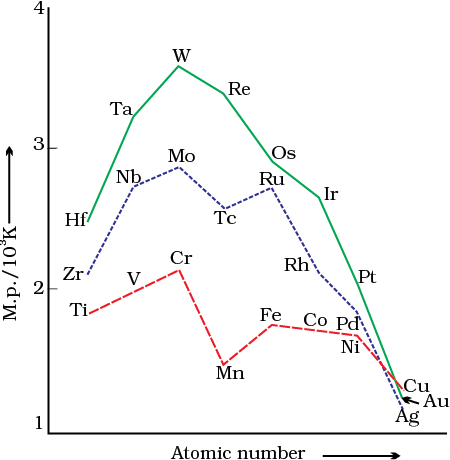
The transition metals (with the exception of Zn, Cd and Hg) are very hard and have low volatility. Their melting and boiling points are high. Fig. 8.1 depicts the melting points of transition metals belonging to 3d, 4d and 5d series. The high melting points of these metals are attributed to the involvement of greater number of electrons from (n-1)d in addition to the ns electrons in the interatomic metallic bonding. In any row the melting points of these metals rise to a maximum at d5 except for anomalous values of Mn and Tc and fall regularly as the atomic number increases. They have high enthalpies of atomisation which are shown in Fig. 8.2. The maxima at about the middle of each series indicate that one unpaired electron per d orbital is particularly favourable for strong interatomic interaction. In general, greater the number of valence electrons, stronger is the resultant bonding. Since the enthalpy of atomisation is an important factor in determining the standard electrode potential of a metal, metals with very high enthalpy of atomisation (i.e., very high boiling point) tend to be noble in their reactions (see later for electrode potentials).
Another generalisation that may be drawn from Fig. 8.2 is that the metals of the second and third series have greater enthalpies of atomisation than the corresponding elements of the first series; this is an important factor in accounting for the occurrence of much more frequent metal – metal bonding in compounds of the heavy transition metals.
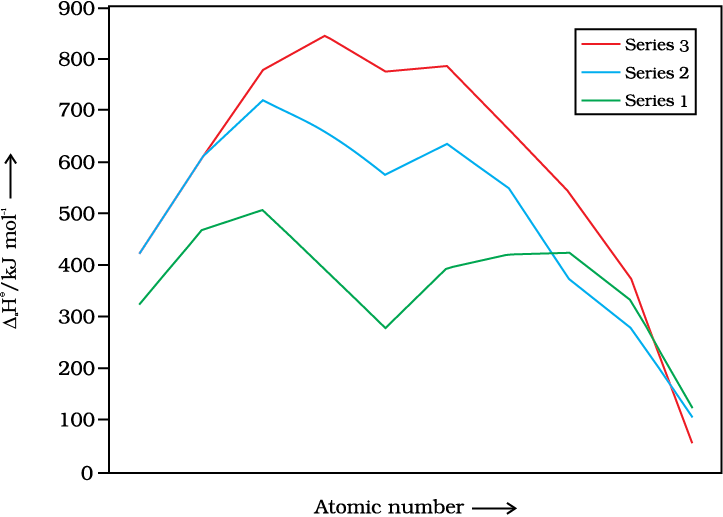
Fig. 8.2 Trends in enthalpies of atomisation of transition elements

© 2026 GoodEd Technologies Pvt. Ltd.