These oxides are generally formed by the reaction of metals with oxygen at high temperatures. All the metals except scandium form MO oxides which are ionic. The highest oxidation number in the oxides, coincides with the group number and is attained in Sc2O3 to Mn2O7. Beyond group 7, no higher oxides of iron above Fe2O3 are known. Besides the oxides, the oxocations stabilise VV as VO2+, VIV as VO2+ and TiIV as TiO2+.
Thus, Mn2O7 gives HMnO4 and CrO3 gives H2CrO4 and H2Cr2O7. V2O5 is, however, amphoteric though mainly acidic and it gives VO43– as well as VO2+ salts. In vanadium there is gradual change from the basic V2O3 to less basic V2O4 and to amphoteric V2O5. V2O4 dissolves in acids to give VO2+ salts. Similarly, V2O5 reacts with alkalies as well as acids to give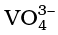 and
and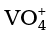 respectively. The well characterised CrO is basic but Cr2O3 is amphoteric.
respectively. The well characterised CrO is basic but Cr2O3 is amphoteric.
Potassium dichromate K2Cr2O7
Potassium dichromate is a very important chemical used in leather industry and as an oxidant for preparation of many azo compounds. Dichromates are generally prepared from chromate, which in turn are obtained by the fusion of chromite ore (FeCr2O4) with sodium or potassium carbonate in free access of air. The reaction with sodium carbonate occurs as follows:
4 FeCr2O4 + 8 Na2CO3 + 7 O2 → 8 Na2CrO4 + 2 Fe2O3 + 8 CO2
The yellow solution of sodium chromate is filtered and acidified with sulphuric acid to give a solution from which orange sodium dichromate, Na2Cr2O7. 2H2O can be crystallised.
2Na2CrO4 + 2 H+ → Na2Cr2O7 + 2 Na+ + H2O
Sodium dichromate is more soluble than potassium dichromate. The latter is therefore, prepared by treating the solution of sodium dichromate with potassium chloride.
Na2Cr2O7 + 2 KCl → K2Cr2O7 + 2 NaCl
Orange crystals of potassium dichromate crystallise out. The chromates and dichromates are interconvertible in aqueous solution depending upon pH of the solution. The oxidation state of chromium in chromate and dichromate is the same.
2 CrO42– + 2H+ → Cr2O72– + H2O
Cr2O72– + 2 OH- → 2 CrO42– + H2O
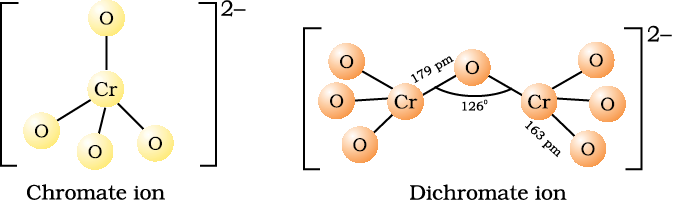
Sodium and potassium dichromates are strong oxidising agents; the sodium salt has a greater solubility in water and is extensively used as an oxidising agent in organic chemistry. Potassium dichromate is used as a primary standard in volumetric analysis. In acidic solution, its oxidising action can be represented as follows:
Cr2O72– + 14H+ + 6e– → 2Cr3+ + 7H2O (EƟ = 1.33V)
Thus, acidified potassium dichromate will oxidise iodides to iodine, sulphides to sulphur, tin(II) to tin(IV) and iron(II) salts to iron(III). The half-reactions are noted below:
6 I– → 3I2 + 6 e–; 3 Sn2+ → 3Sn4+ + 6 e–
3 H2S → 6H+ + 3S + 6e–; 6 Fe2+ → 6Fe3+ + 6 e–
The full ionic equation may be obtained by adding the half-reaction for potassium dichromate to the half-reaction for the reducing agent, for e.g.,
Cr2O72– + 14 H+ + 6 Fe2+ → 2 Cr3+ + 6 Fe3+ + 7 H2O
Potassium permanganate KMnO4
Potassium permanganate is prepared by fusion of MnO2 with an alkali metal hydroxide and an oxidising agent like KNO3. This produces the dark green K2MnO4 which disproportionates in a neutral or acidic solution to give permanganate.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
3MnO42– + 4H+ → 2MnO4– + MnO2 + 2H2O
Commercially it is prepared by the alkaline oxidative fusion of MnO2 followed by the electrolytic oxidation of manganate (Vl).
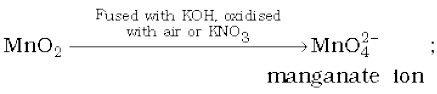

In the laboratory, a manganese (II) ion salt is oxidised by peroxodisulphate to permanganate.
2Mn2+ + 5S2O82– + 8H2O → 2MnO4– + 10SO42– + 16H+
Potassium permanganate forms dark purple (almost black) crystals which are isostructural with those of KClO4. The salt is not very soluble in water (6.4 g/100 g of water at 293 K), but when heated it decomposes at 513 K.
2KMnO4 → K2MnO4 + MnO2 + O2
It has two physical properties of considerable interest: its intense colour and its diamagnetism along with temperature-dependent weak paramagnetism. These can be explained by the use of molecular orbital theory which is beyond the present scope.
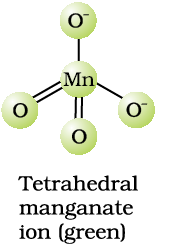
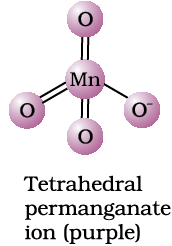
The manganate and permanganate ions are tetrahedral; the π-bonding takes place by overlap of p orbitals of oxygen with d orbitals of manganese. The green manganate is paramagnetic because of one unpaired electron but the permanganate is diamagnetic due to the absence of unpaired electron.
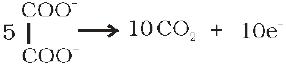
5 Fe2+ → 5 Fe3+ + 5e–
5NO2– + 5H2O → 5NO3– + 10H+ + l0e–
10I– → 5I2 + 10e–
The full reaction can be written by adding the half-reaction for KMnO4 to the half-reaction of the reducing agent, balancing wherever necessary.
If we represent the reduction of permanganate to manganate, manganese dioxide and manganese(II) salt by half-reactions,
MnO4– + e– → MnO42– (EƟ = + 0.56 V)
MnO4– + 4H+ + 3e– → MnO2 + 2H2O (EƟ = + 1.69 V)
MnO4– + 8H+ + 5e– → Mn2+ + 4H2O (EƟ = + 1.52 V)
A few important oxidising reactions of KMnO4 are given below:
1. In acid solutions:
(a) Iodine is liberated from potassium iodide :
10I– + 2MnO4– + 16H+ → 2Mn2+ + 8H2O + 5I2
(b) Fe2+ ion (green) is converted to Fe3+ (yellow):
5Fe2+ + MnO4– + 8H+ → Mn2+ + 4H2O + 5Fe3+
(c) Oxalate ion or oxalic acid is oxidised at 333 K:
5C2O42– + 2MnO4– + 16H+ ——> 2Mn2+ + 8H2O + 10CO2
(d) Hydrogen sulphide is oxidised, sulphur being precipitated:
H2S —> 2H+ + S2–
5S2– + 2MnO–4 + 16H+ ——> 2Mn2+ + 8H2O + 5S
(e) Sulphurous acid or sulphite is oxidised to a sulphate or sulphuric acid:
5SO32– + 2MnO4– + 6H+ ——> 2Mn2+ + 3H2O + 5SO42–
(f) Nitrite is oxidised to nitrate:
5NO2– + 2MnO4– + 6H+ ——> 2Mn2+ + 5NO3– + 3H2O
2. In neutral or faintly alkaline solutions:
(a) A notable reaction is the oxidation of iodide to iodate:
2MnO4– + H2O + I– ——> 2MnO2 + 2OH– + IO3–
(b) Thiosulphate is oxidised almost quantitatively to sulphate:
8MnO4– + 3S2O32– + H2O ——> 8MnO2 + 6SO42– + 2OH–
(c) Manganous salt is oxidised to MnO2; the presence of zinc sulphate or zinc oxide catalyses the oxidation:
2MnO4– + 3Mn2+ + 2H2O ——> 5MnO2 + 4H+
Note: Permanganate titrations in presence of hydrochloric acid are unsatisfactory since hydrochloric acid is oxidised to chlorine.
Uses: Besides its use in analytical chemistry, potassium permanganate is used as a favourite oxidant in preparative organic chemistry. Its uses for the bleaching of wool, cotton, silk and other textile fibres and for the decolourisation of oils are also dependent on its strong oxidising power.
The Inner Transition Elements (f -BLOCK)
The f-block consists of the two series, lanthanoids (the fourteen elements following lanthanum) and actinoids (the fourteen elements following actinium). Because lanthanum closely resembles the lanthanoids, it is usually included in any discussion of the lanthanoids for which the general symbol Ln is often used. Similarly, a discussion of the actinoids includes actinium besides the fourteen elements constituting the series. The lanthanoids resemble one another more closely than do the members of ordinary transition elements in any series. They have only one stable oxidation state and their chemistry provides an excellent opportunity to examine the effect of small changes in size and nuclear charge along a series of otherwise similar elements. The chemistry of the actinoids is, on the other hand, much more complicated. The complication arises partly owing to the occurrence of a wide range of oxidation states in these elements and partly because their radioactivity creates special problems in their study; the two series will be considered separately here.