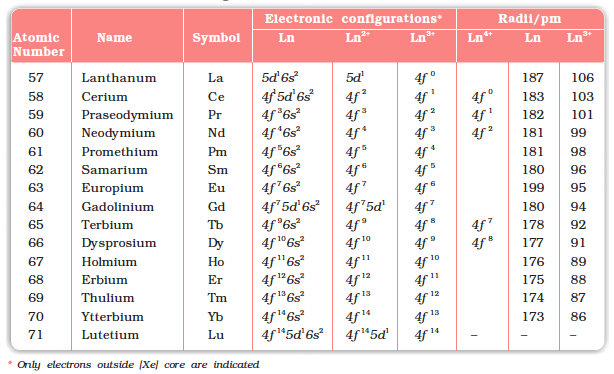
The names, symbols, electronic configurations of atomic and some ionic states and atomic and ionic radii of lanthanum and lanthanoids (for which the general symbol Ln is used) are given in Table 8.9.
It may be noted that atoms of these elements have electronic configuration with 6s2 common but with variable occupancy of 4f level (Table 8.9). However, the electronic configurations of all the tripositive ions (the most stable oxidation state of all the lanthanoids) are of the form 4f n (n = 1 to 14 with increasing atomic number).
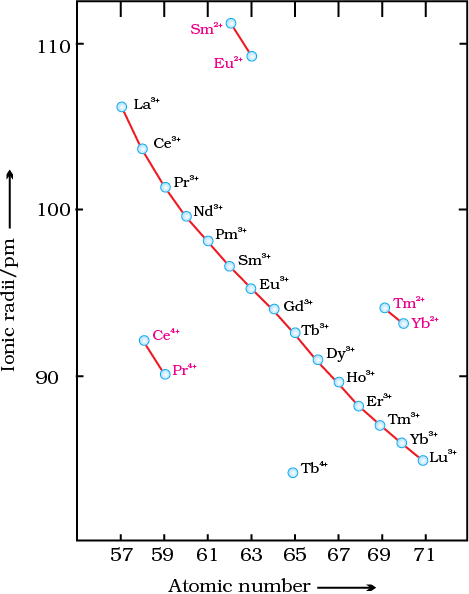
Fig. 8.6: Trends in ionic radii of lanthanoids
The cumulative effect of the contraction of the lanthanoid series, known as lanthanoid contraction, causes the radii of the members of the third transition series to be very similar to those of the corresponding members of the second series. The almost identical radii of Zr (160 pm) and Hf (159 pm), a consequence of the lanthanoid contraction, account for their occurrence together in nature and for the difficulty faced in their separation.
In the lanthanoids, La(II) and Ln(III) compounds are predominant species. However, occasionally +2 and +4 ions in solution or in solid compounds are also obtained. This irregularity (as in ionisation enthalpies) arises mainly from the extra stability of empty, half-filled or filled f subshell. Thus, the formation of CeIV is favoured by its noble gas configuration, but it is a strong oxidant reverting to the common +3 state. The Eo value for Ce4+/ Ce3+ is + 1.74 V which suggests that it can oxidise water. However, the reaction rate is very slow and hence Ce(IV) is a good analytical reagent. Pr, Nd, Tb and Dy also exhibit +4 state but only in oxides, MO2. Eu2+ is formed by losing the two s electrons and its f 7 configuration accounts for the formation of this ion. However, Eu2+ is a strong reducing agent changing to the common +3 state. Similarly Yb2+ which has f 14 configuration is a reductant. TbIV has half-filled f-orbitals and is an oxidant. The behaviour of samarium is very much like europium, exhibiting both +2 and +3 oxidation states.
Table 8.9: Electronic Configurations and Radii of Lanthanum and Lanthanoids