Decacarbonyldimanganese(0) is made up of two square pyramidal Mn(CO)5 units joined by a Mn – Mn bond. Octacarbonyldicobalt(0) has a Co – Co bond bridged by two CO groups (Fig.9.13).
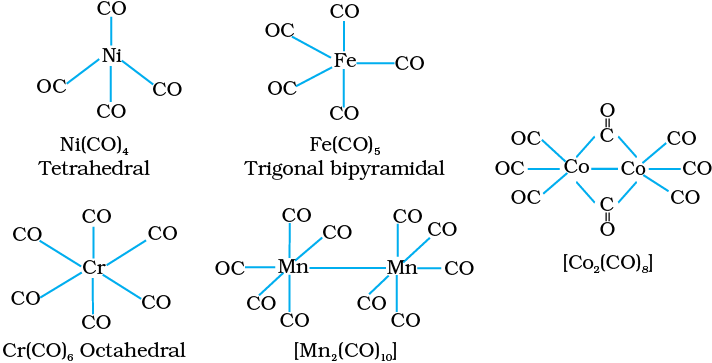
Fig. 9.13 Structures of some representative homoleptic metal carbonyls.
The metal-carbon bond in metal carbonyls possess both σ and π character. The M–C σ bond is formed by the donation of lone pair of electrons on the carbonyl carbon into a vacant orbital of the metal. The M–C π bond is formed by the donation of a pair of electrons from a filled d orbital of metal into the vacant antibonding π* orbital of carbon monoxide. The metal to ligand bonding creates a synergic effect which strengthens the bond between CO and the metal (Fig.9.14).
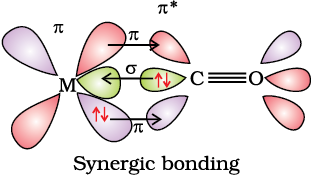
Fig. 9.14: Example of synergic bonding interactions in a carbonyl complex.
The stability of a complex in solution refers to the degree of association between the two species involved in the state of equilibrium. The magnitude of the (stability or formation) equilibrium constant for the association, quantitatively expresses the stability. Thus, if we have a reaction of the type:
then the larger the stability constant, the higher the proportion of ML4 that exists in solution. Free metal ions rarely exist in the solution so that M will usually be surrounded by solvent molecules which will compete with the ligand molecules, L, and be successively replaced by them. For simplicity, we generally ignore these solvent molecules and write four stability constants as follows:
where etc., are referred to as stepwise stability constants. Alternatively, we can write the overall stability constant thus:
The stepwise and overall stability constant is therefore related as follows:
or more generally,
If we take as an example, the steps involved in the formation of the cuprammonium ion, we have the following:
where are the stepwise stability constants and overall stability constant.
Also
The addition of the four amine groups to copper shows a pattern found for most formation constants, in that the successive stability constants decrease. In this case, the four constants are:
The instability constant or the dissociation constant of coordination compounds is defined as the reciprocal of the formation constant.
Intext Question
9.11 Calculate the overall complex dissociation equilibrium constant for the ion, given that
The coordination compounds are of great importance. These compounds are widely present in the mineral, plant and animal worlds and are known to play many important functions in the area of analytical chemistry, metallurgy, biological systems, industry and medicine. These are described below:
• Coordination compounds find use in many qualitative and quantitative chemical analysis. The familiar colour reactions given by metal ions with a number of ligands (especially chelating ligands), as a result of the formation of coordination entities, form the basis for their detection and estimation by classical and instrumental methods of analysis. Examples of such reagents include EDTA, DMG (dimethylglyoxime), α–nitroso–β–naphthol, coupon, etc.
• Hardness of water is estimated by simple titration with Na2EDTA. The Ca2+ and Mg2+ ions form stable complexes with EDTA. The selective estimation of these ions can be done due to the difference in the stability constants of calcium and magnesium complexes.
• Some important extraction processes of metals, like those of silver and gold, make use of complex formation. Gold, for example, combines with cyanide in the presence of oxygen and water to form the coordination entity [Au(CN)2]– in aqueous solution. Gold can be separated in metallic form from this solution by the addition of zinc (Unit 6).
• Similarly, purification of metals can be achieved through formation and subsequent decomposition of their coordination compounds. For example, impure nickel is converted to [Ni(CO)4], which is decomposed to yield pure nickel.
• Coordination compounds are used as catalysts for many industrial processes. Examples include rhodium complex, [(Ph3P)3RhCl], a Wilkinson catalyst, is used for the hydrogenation of alkenes.
• Articles can be electroplated with silver and gold much more smoothly and evenly from solutions of the complexes, [Ag(CN)2]– and [Au(CN)2]– than from a solution of simple metal ions.
• In black and white photography, the developed film is fixed by washing with hypo solution which dissolves the undecomposed AgBr to form a complex ion, [Ag(S2O3)2]3–.
• There is growing interest in the use of chelate therapy in medicinal chemistry. An example is the treatment of problems caused by the presence of metals in toxic proportions in plant/animal systems. Thus, excess of copper and iron are removed by the chelating ligands D–penicillamine and desferrioxamine B via the formation of coordination compounds. EDTA is used in the treatment of lead poisoning. Some coordination compounds of platinum effectively inhibit the growth of tumours. Examples are cisplatin and related compounds.