Having learnt the classification of halogenated compounds, let us now learn how these are named. The common names of alkyl halides are derived by naming the alkyl group followed by the name of halide. In the IUPAC system of nomenclature, alkyl halides are named as halosubstituted hydrocarbons. For mono halogen substituted derivatives of benzene, common and IUPAC names are the same. For dihalogen derivatives, the prefixes o-, m-, p- are used in common system but in IUPAC system, as you have learnt in Class XI, Unit 12, the numerals 1,2; 1,3 and 1,4 are used.
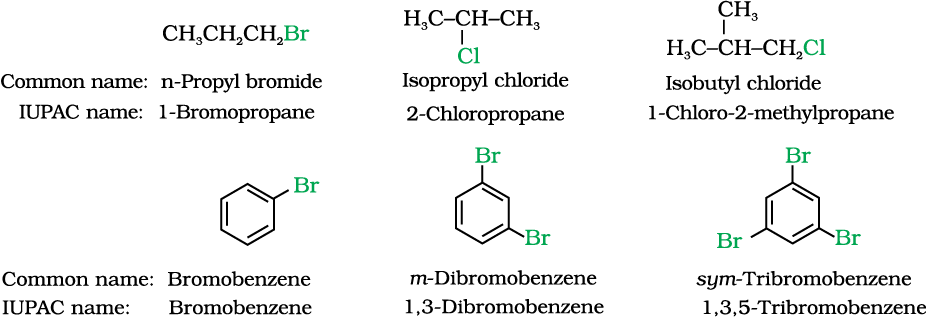
The dihaloalkanes having the same type of halogen atoms are named as alkylidene or alkylene dihalides. The dihalo-compounds having both the halogen atoms are further classified as geminal halides or gem-dihalides when both the halogen atoms are present on the same carbon atom of the chain and vicinal halides or vic-dihalides when halogen atoms are present on adjacent carbon atoms. In common name system, gem-dihalides are named as alkylidene halides and vic-dihalides are named as alkylene dihalides. In IUPAC system, they are named as dihaloalkanes.
Table 10.1: Common and IUPAC Names of some Halides
Example 10.1
Draw the structures of all the eight structural isomers that have the molecular formula C5H11Br. Name each isomer according to IUPAC system and classify them as primary, secondary or tertiary bromide.
Solution
CH3CH2CH2CH2CH2Br 1-Bromopentane (1°)
CH3CH2CH2CH(Br)CH3 2-Bromopentane(2°)
CH3CH2CH(Br)CH2CH3 3-Bromopentane (2°)
(CH3)2CHCH2CH2Br 1-Bromo-3-methylbutane (1°)
(CH3)2CHCHBrCH3 2-Bromo-3-methylbutane(2°)
(CH3)2CBrCH2CH3 2-Bromo-2-methylbutane (3°)
CH3CH2CH(CH3)CH2Br 1-Bromo-2-methylbutane(1°)
(CH3)3CCH2Br 1-Bromo-2,2-dimethylpropane (1°)
Example
Write IUPAC names of the following:
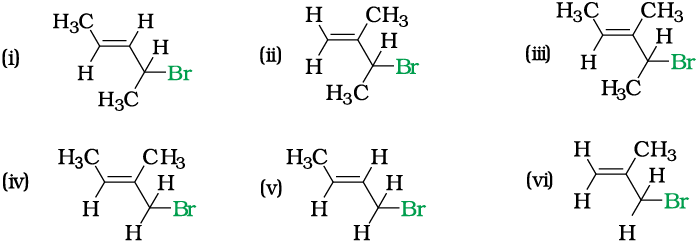
Solution
(i) 4-Bromopent-2-ene (ii) 3-Bromo-2-methylbut-1-ene
(iii) 4-Bromo-3-methylpent-2-ene (iv) 1-Bromo-2-methylbut-2-ene
(v) 1-Bromobut-2-ene (vi) 3-Bromo-2-methylpropene
Intext Question
10.1 Write structures of the following compounds:
(i) 2-Chloro-3-methylpentane
(ii) 1-Chloro-4-ethylcyclohexane
(iii) 4-tert. Butyl-3-iodoheptane
(iv) 1,4-Dibromobut-2-ene
(v) 1-Bromo-4-sec. butyl-2-methylbenzene.