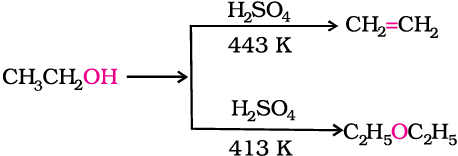
1. By dehydration of alcohols
Alcohols undergo dehydration in the presence of protic acids (H2SO4, H3PO4). The formation of the reaction product, alkene or ether depends on the reaction conditions. For example, ethanol is dehydrated to ethene in the presence of sulphuric acid at 443 K. At 413 K, ethoxyethane is the main product.
Diethyl ether has been used widely as an inhalation anaesthetic. But due to its slow effect and an unpleasant recovery period, it has been replaced, as an anaesthetic, by other compounds.
The formation of ether is a nucleophilic bimolecular reaction (Sn2) involving the attack of alcohol molecule on a protonated alcohol, as indicated below:
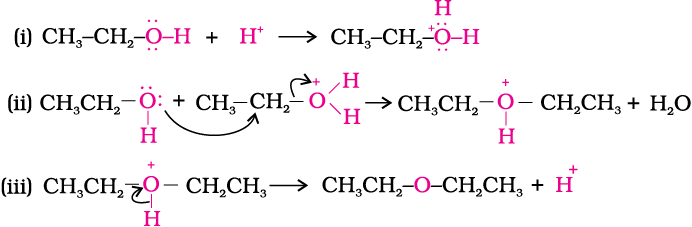
Acidic dehydration of alcohols, to give an alkene is also associated with substitution reaction to give an ether.
The method is suitable for the preparation of ethers having primary alkyl groups only. The alkyl group should be unhindered and the temperature be kept low. Otherwise the reaction favours the formation of alkene. The reaction follows SN1 pathway when the alcohol is secondary or tertiary about which you will learn in higher classes. However, the dehydration of secondary and tertiary alcohols to give corresponding ethers is unsuccessful as elimination competes over substitution and as a consequence, alkenes are easily formed.
Can you explain why is bimolecular dehydration not appropriate for the preparation of ethyl methyl ether?
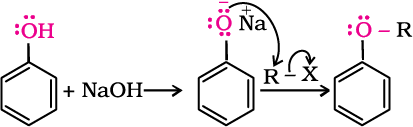
2. Williamson synthesis
It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers. In this method, an alkyl halide is allowed to react with sodium alkoxide.
Ethers containing substituted alkyl groups (secondary or tertiary) may also be prepared by this method. The reaction involves Sn2 attack of an alkoxide ion on primary alkyl halide.
Alexander William Williamson (1824–1904) was born in London of Scottish parents. In 1849, he became Professor of Chemistry at University College, London.
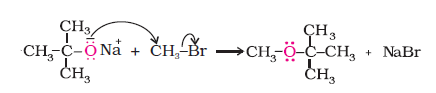
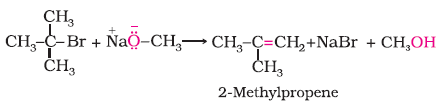
Example 11.6
The following is not an appropriate reaction for the preparation of t-butyl ethyl ether.
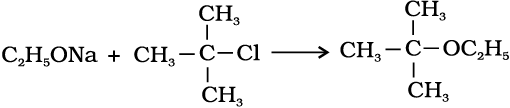
(i) What would be the major product of this reaction ?
(ii) Write a suitable reaction for the preparation of t-butylethyl ether.
Solution
(i) The major product of the given reaction is 2-methylprop-1-ene. It is because sodium ethoxide is a strong nucleophile as well as a strong base. Thus elimination reaction predominates over substitution.
(ii) 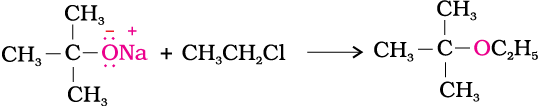
Phenols are also converted to ethers by this method. In this, phenol is used as the phenoxide moiety.