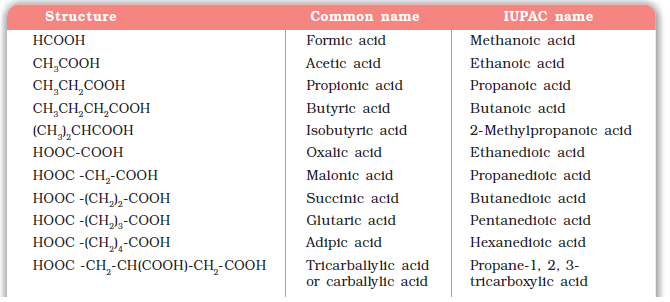
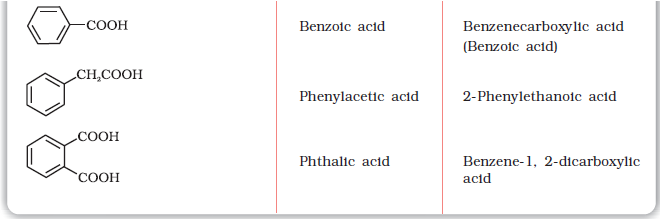
Since carboxylic acids are amongst the earliest organic compounds to be isolated from nature, a large number of them are known by their common names. The common names end with the suffix –ic acid and have been derived from Latin or Greek names of their natural sources. For example, formic acid (HCOOH) was first obtained from red ants (Latin: formica means ant), acetic acid (CH3COOH) from vinegar (Latin: acetum, means vinegar), butyric acid (CH3CH2CH2COOH) from rancid butter (Latin: butyrum, means butter).
Table 12.3 Names and Structures of Some Carboxylic Acids
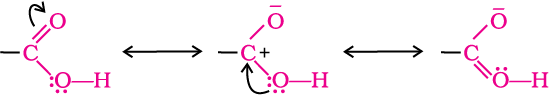
In carboxylic acids, the bonds to the carboxyl carbon lie in one plane and are separated by about 120°. The carboxylic carbon is less electrophilic than carbonyl carbon because of the possible resonance structure shown below:
Intext Question
12.6 Give the IUPAC names of the following compounds:
(i) Ph CH2CH2COOH (ii) (CH3)2C=CHCOOH
(iii) 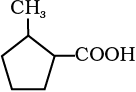 (iv)
(iv) 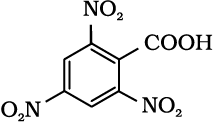

© 2026 GoodEd Technologies Pvt. Ltd.