1. Reduction
Carboxylic acids are reduced to primary alcohols by lithium aluminium hydride or better with diborane. Diborane does not easily reduce functional groups such as ester, nitro, halo, etc. Sodium borohydride does not reduce the carboxyl group.

2. Decarboxylation
Carboxylic acids lose carbon dioxide to form hydrocarbons when their sodium salts are heated with sodalime (NaOH and CaO in the ratio of 3 : 1). The reaction is known as decarboxylation.
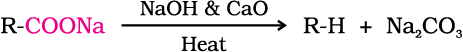
Alkali metal salts of carboxylic acids also undergo decarboxylation on electrolysis of their aqueous solutions and form hydrocarbons having twice the number of carbon atoms present in the alkyl group of the acid. The reaction is known as Kolbe electrolysis (Unit 13, Class XI).
12.9.4. Substitution Reactions in the Hydrocarbon Part
1. Halogenation

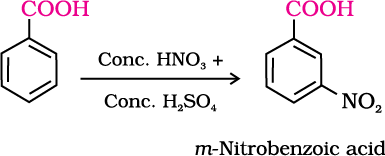
2. Ring substitution
Aromatic carboxylic acids undergo electrophilic substitution reactions in which the carboxyl group acts as a deactivating and meta-directing group. They however, do not undergo Friedel-Crafts reaction (because the carboxyl group is deactivating and the catalyst aluminium chloride (Lewis acid) gets bonded to the carboxyl group).
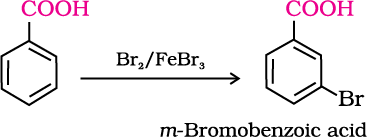
Intext Question
12.8 Which acid of each pair shown here would you expect to be stronger?
(i) CH3CO2H or CH2FCO2H
(ii) CH2FCO2H or CH2ClCO2H
(iii) CH2FCH2CH2CO2H or CH3CHFCH2CO2H
(iv)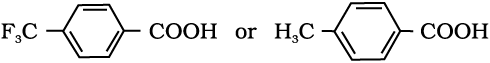

© 2026 GoodEd Technologies Pvt. Ltd.